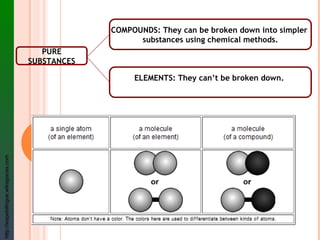
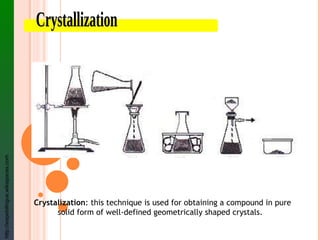
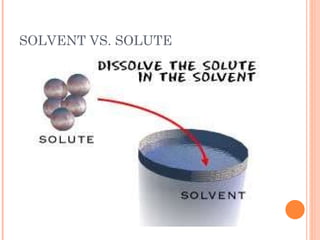
The document provides information about classifying mixtures as pure substances, homogeneous mixtures, or heterogeneous mixtures. It discusses the key characteristics of different types of mixtures including solutions, suspensions, and colloids. Examples are given of each type of mixture along with separation techniques used to distinguish between pure substances and different kinds of mixtures. Key terms are defined and students are assigned a vocabulary activity using those terms.