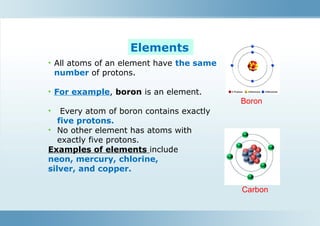
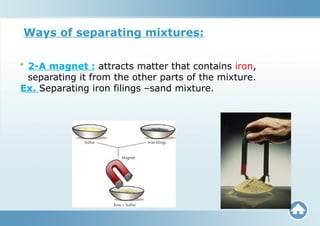
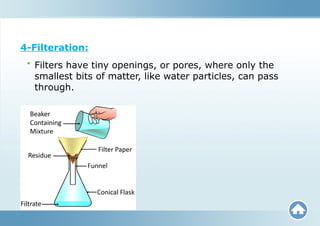
The document describes substances, including elements (pure substances made of one kind of atom) and compounds (formed from the combination of different atoms). It distinguishes between mixtures, which are blends of substances not chemically bonded, and outlines the types of mixtures (heterogeneous and homogeneous). Furthermore, it explains how compounds and mixtures differ in terms of properties and methods of separation.