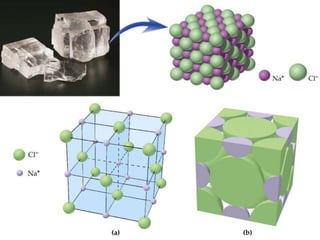
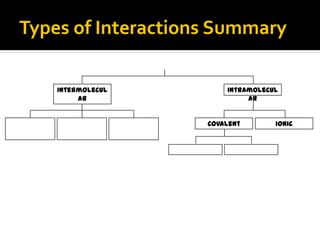
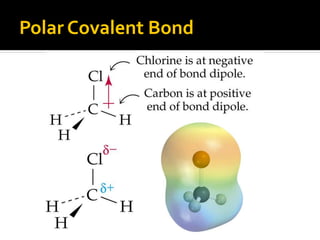
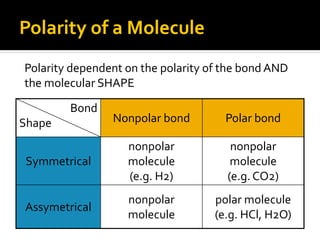
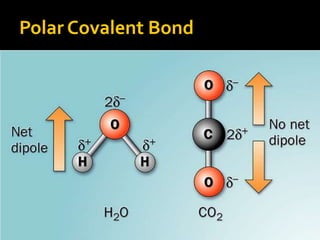
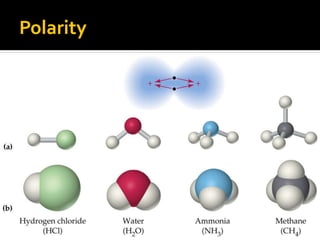
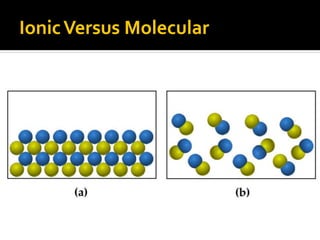
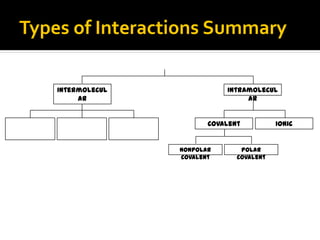
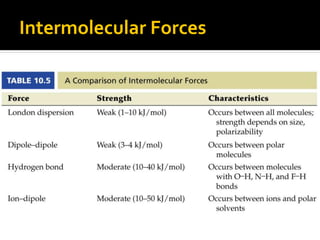
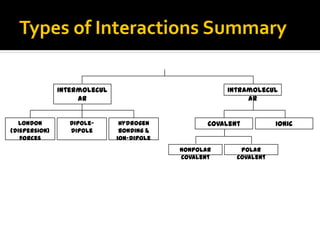
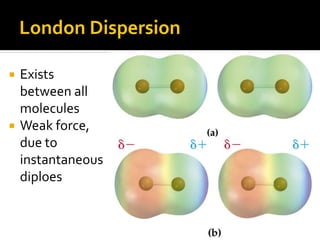
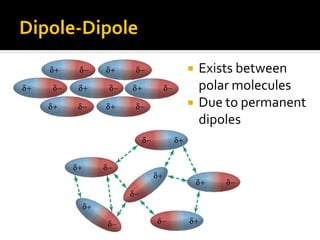
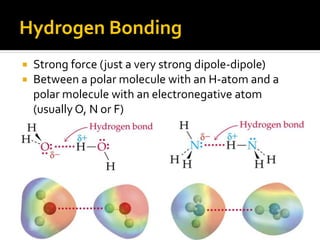
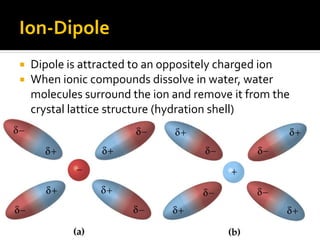
The document defines intramolecular and intermolecular bonding. Intramolecular bonding occurs within a molecule via ionic bonds between atoms with different electronegativity or covalent bonds where atoms share or unequally share electrons. Intermolecular bonding occurs between molecules via weaker London dispersion forces, dipole-dipole interactions, hydrogen bonding, ion-dipole interactions, or hydrophobic interactions. The document provides examples of different types of intramolecular and intermolecular bonds.