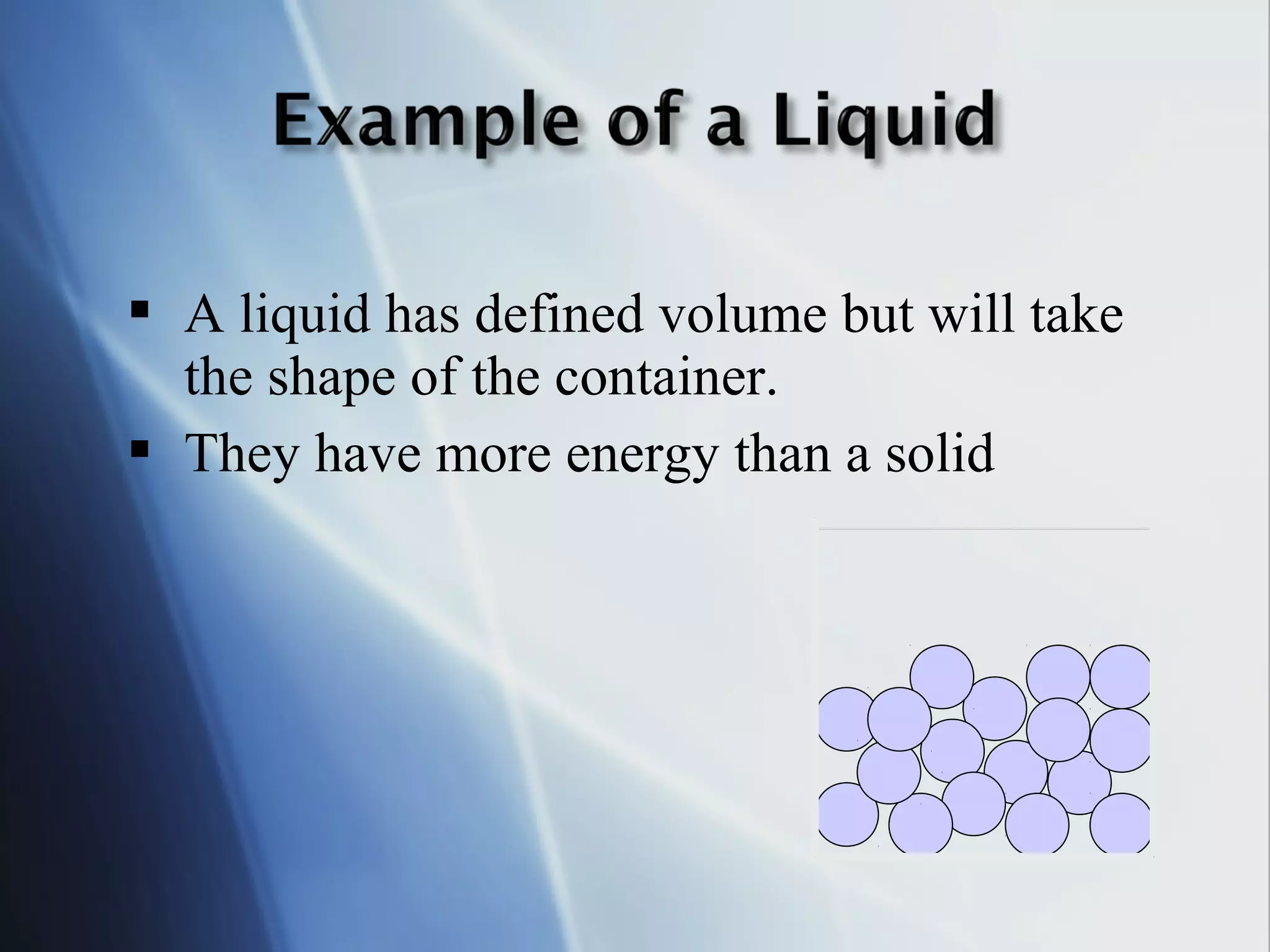
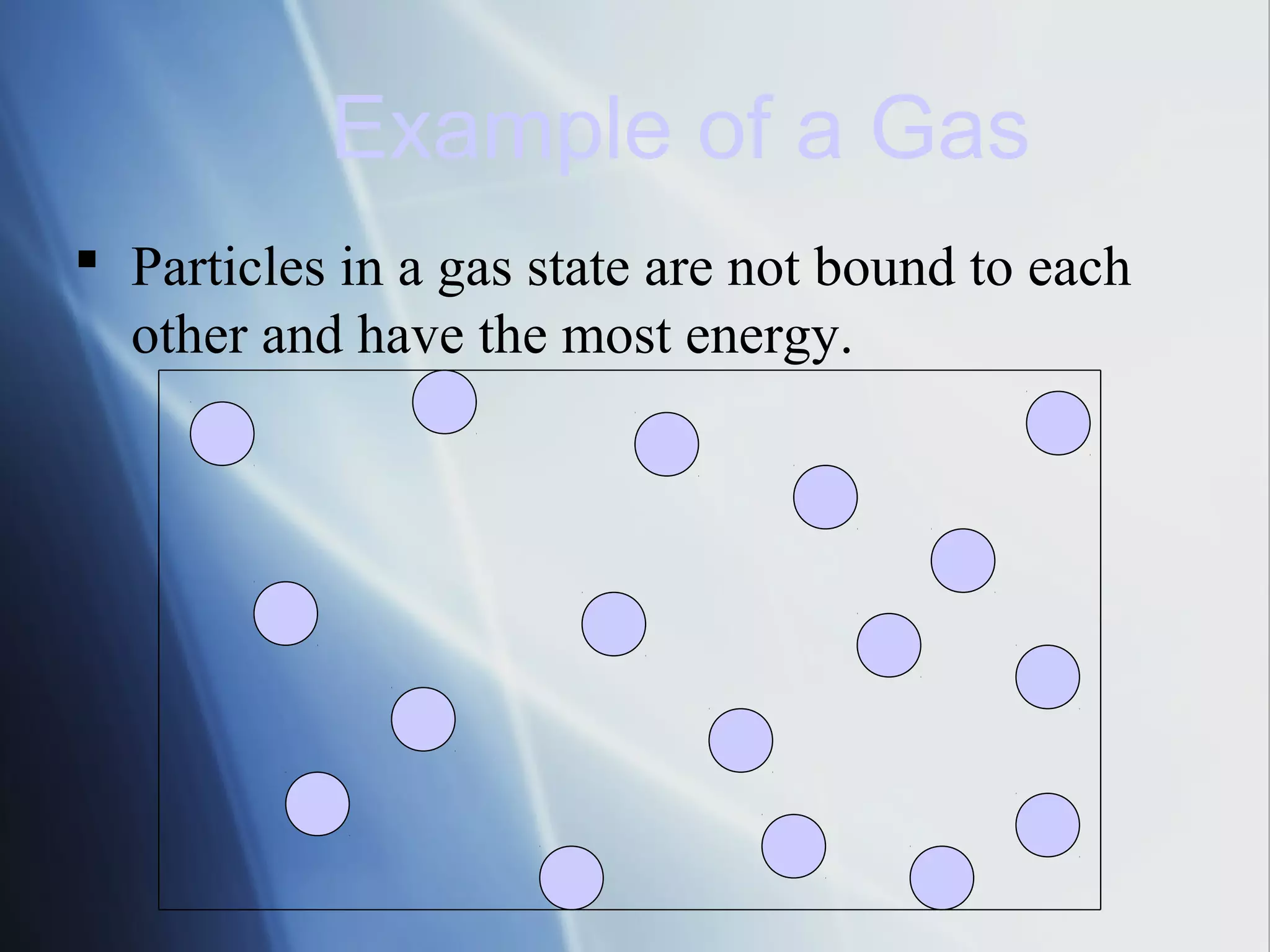
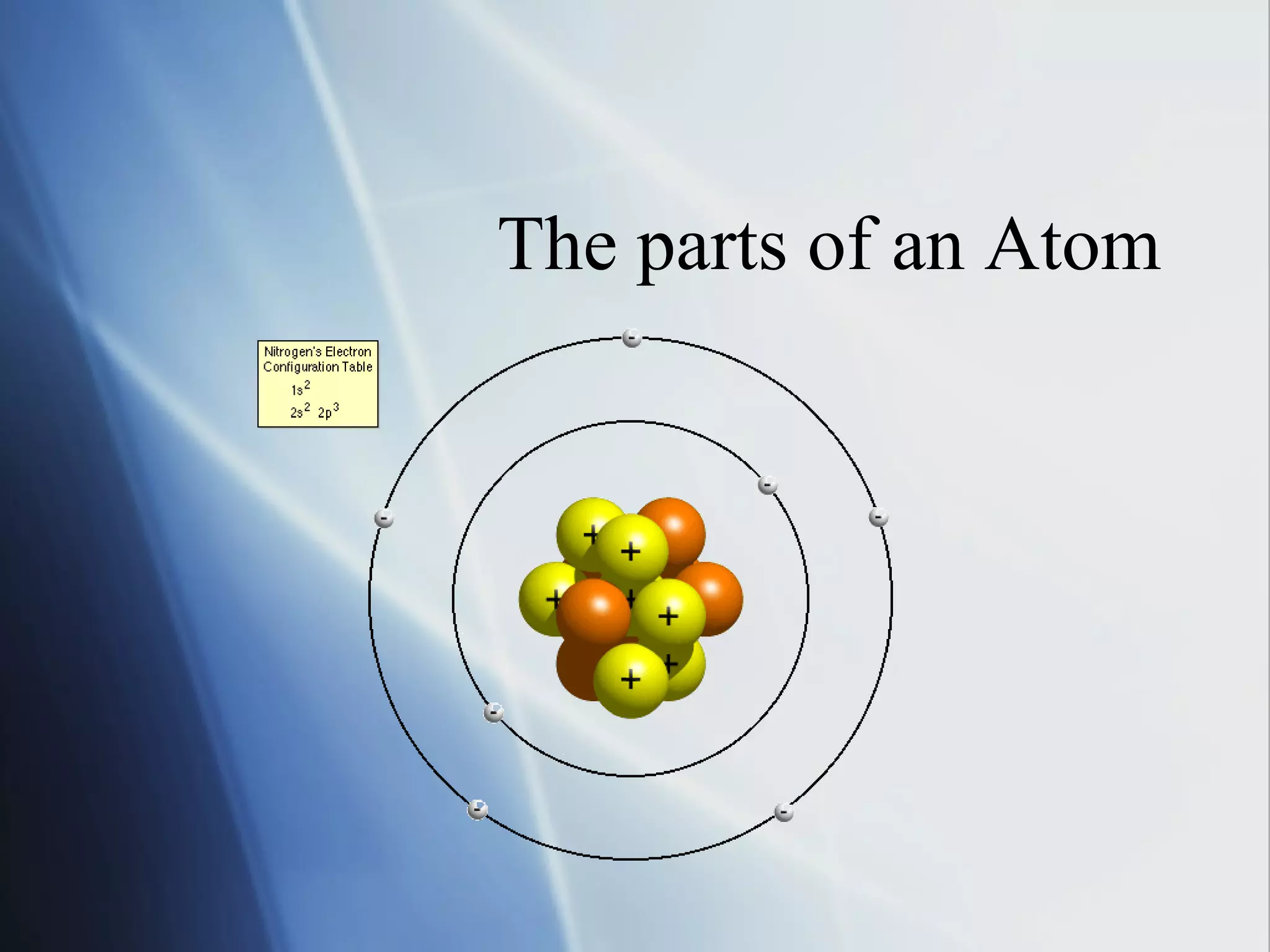
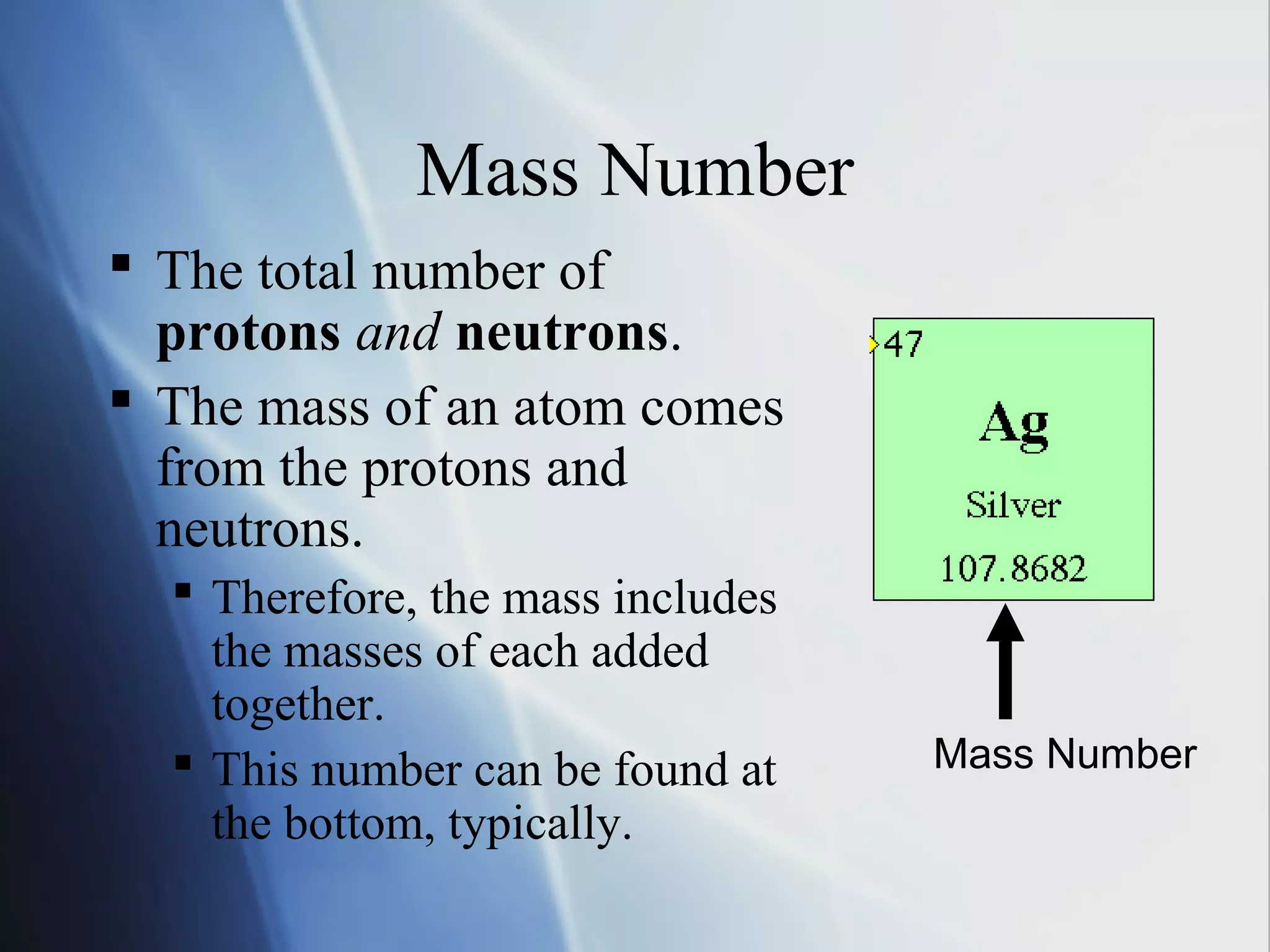
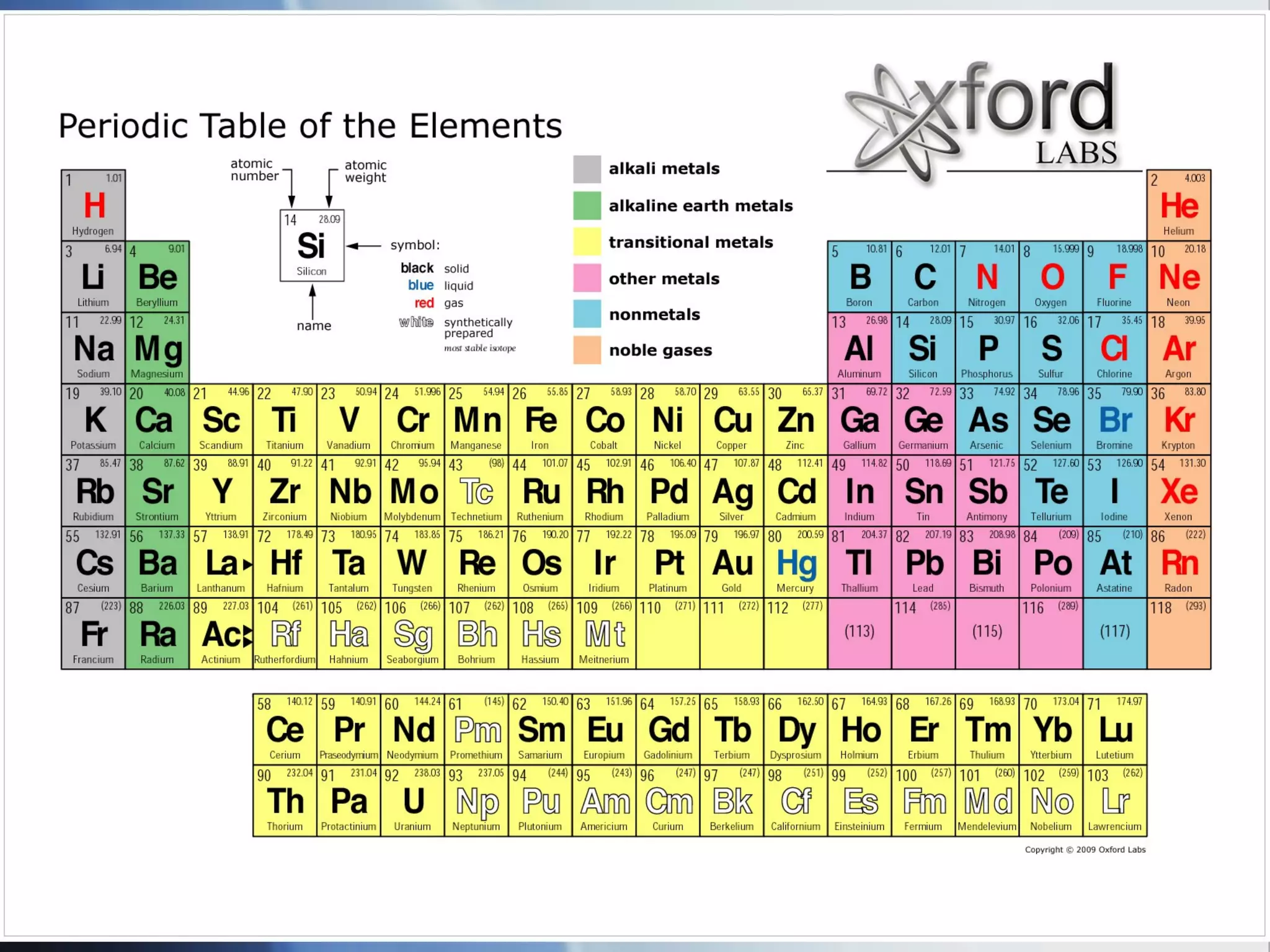
This document provides an overview of 8th grade science concepts related to the structure of matter and states of matter. It explains that all matter is made of particles that vibrate with different amounts of energy depending on their state. Solids have particles with high attraction that vibrate in place, while liquids have less attraction allowing particles to slide past each other, and gases have particles moving independently with empty space between them. Phase changes between solid, liquid and gas are also summarized, including that they require gaining or losing energy and have specific melting, freezing, boiling, and evaporation points or conditions. The document concludes with summaries of atomic structure including electrons, protons, neutrons and energy levels, as well as ionic, covalent and