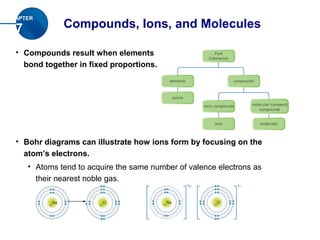
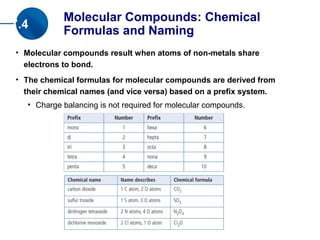
The document discusses compounds, ions, and molecules. It explains that compounds form when elements bond together, and that bonding can occur through electron transfer (ionic bonds) or electron sharing (covalent bonds). Ionic bonding forms ionic compounds, while covalent bonding forms molecular compounds. The document also covers how to write chemical formulas for ionic and molecular compounds based on ion charge balances or molecular prefixes, respectively.