Embed presentation
Downloaded 56 times











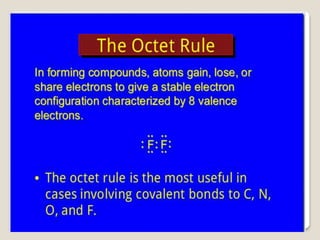
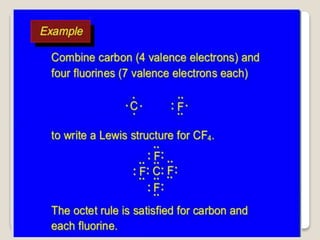
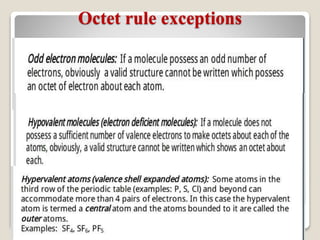


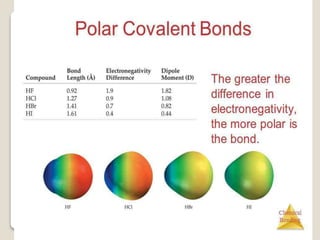
This document discusses several topics related to chemical bonding: Covalent bonding occurs when atoms share valence electrons to form stable molecular structures. Lewis structures use dots to represent these shared and unshared electron pairs, following the octet rule which states that atoms seek a full outer shell of 8 electrons. Exceptions exist for some atoms. Polar covalent bonds form when electrons are unequally shared between atoms, resulting in a partial positive and negative charge.

















