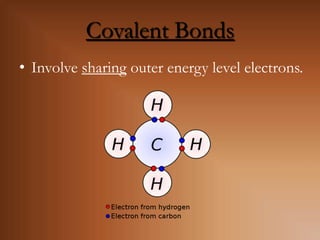
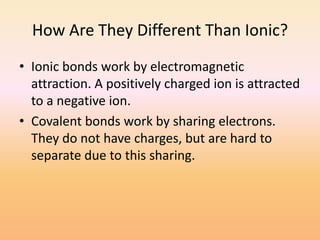
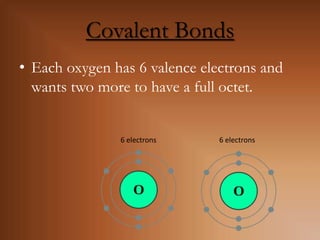
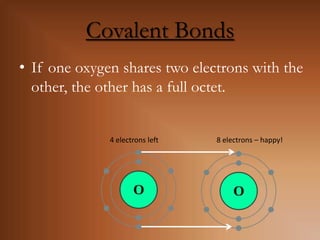
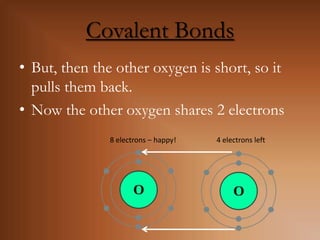
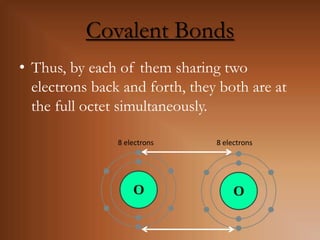
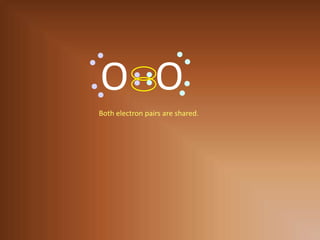
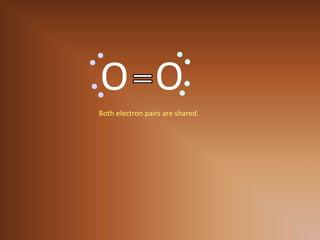
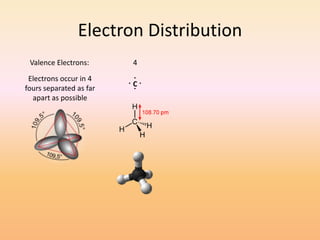
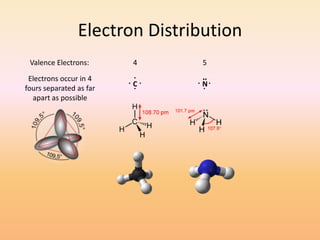
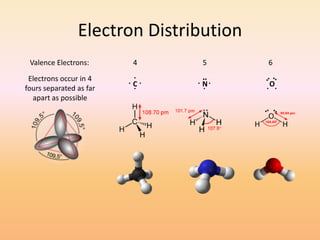
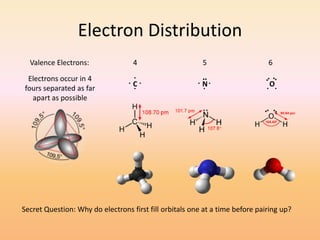
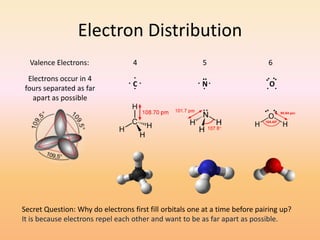
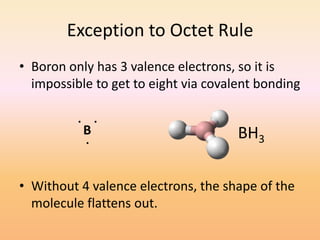
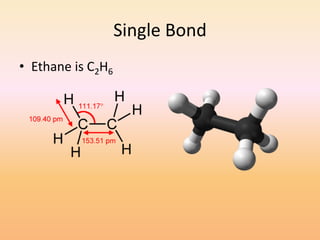
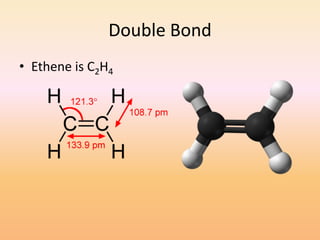
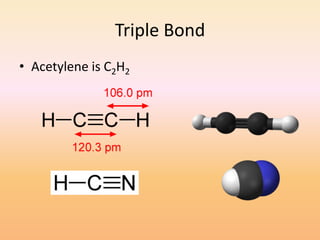
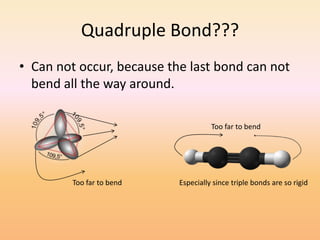
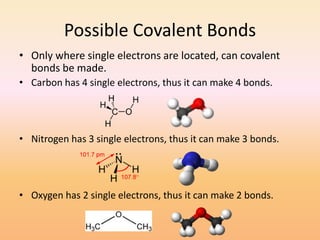
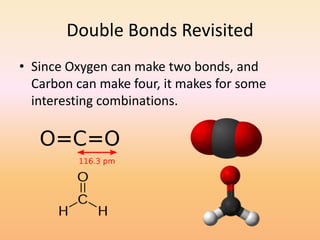
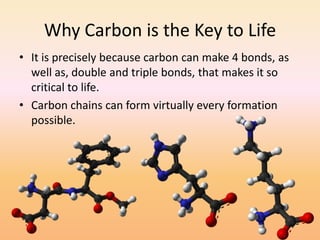
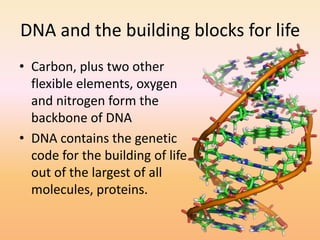
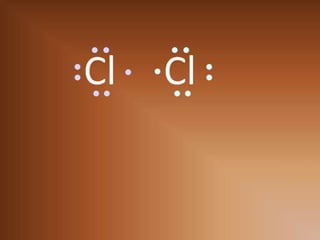This document discusses covalent bonds and how they differ from ionic bonds. Covalent bonds involve sharing electrons between atoms rather than transferring electrons. Chlorine atoms form a covalent bond by each atom sharing an electron pair, allowing both atoms to achieve a full outer shell or octet of electrons. Covalent bonds typically form between nonmetal atoms in groups 3-6 of the periodic table, which have incomplete outer shells and can share electrons to achieve full shells. Carbon is particularly important for life because it can form up to four covalent bonds, allowing for complex carbon chains and structures like DNA and proteins.