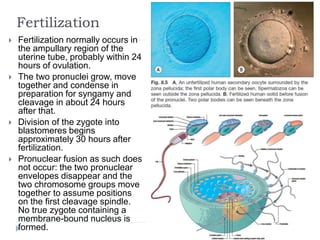
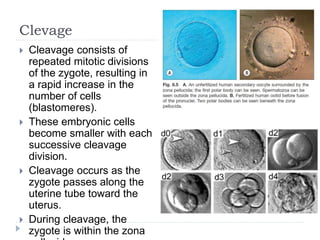
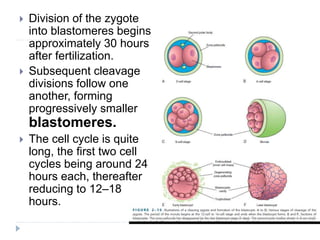
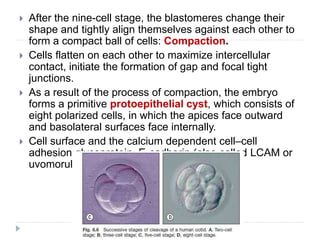
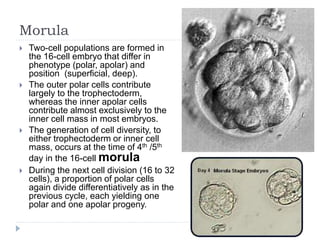
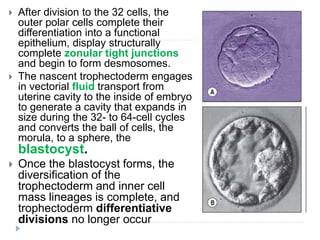
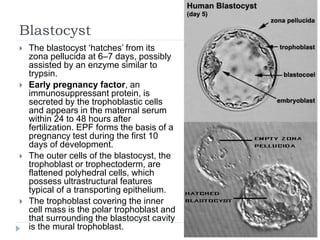
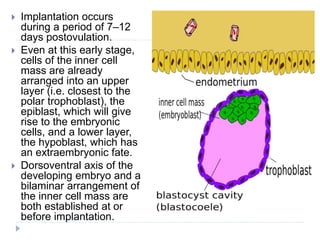
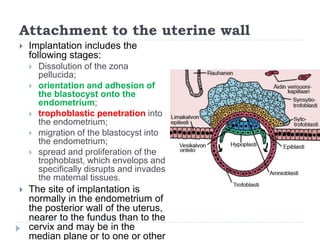
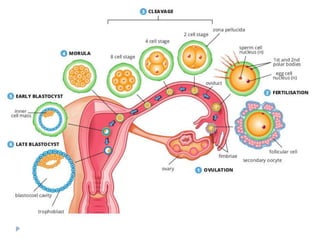
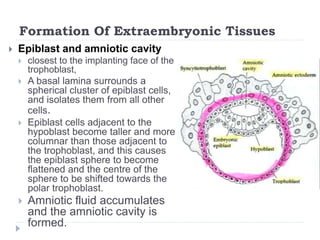
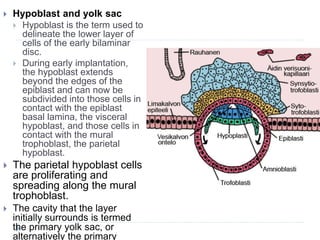
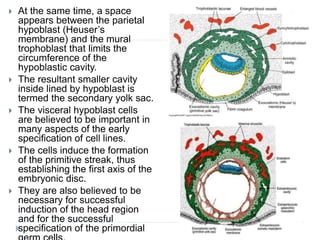
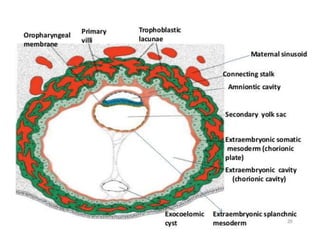
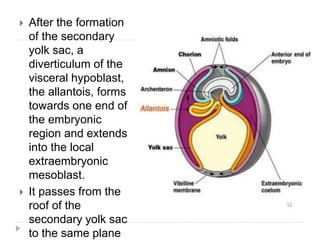
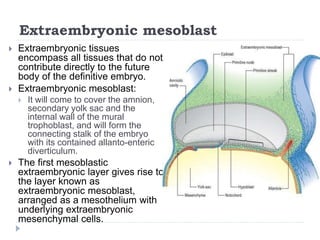
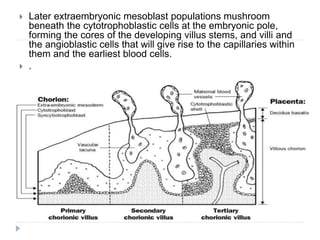
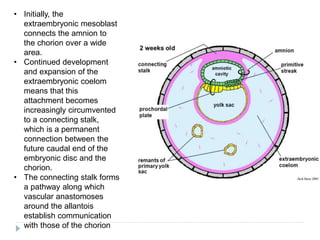
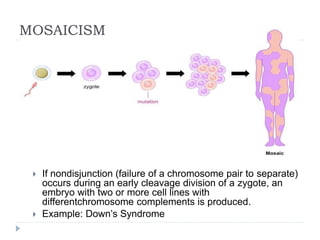
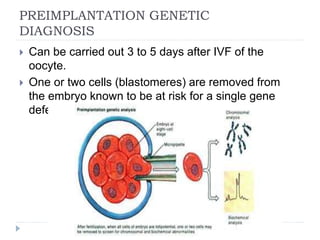
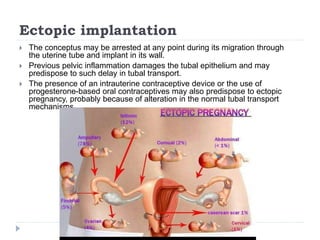
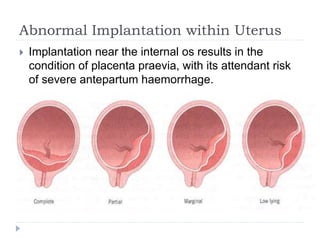
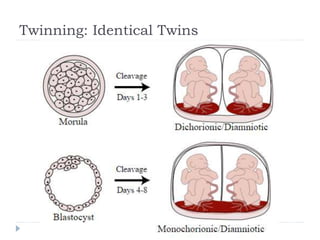
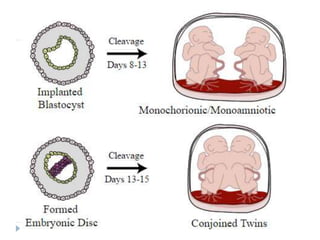
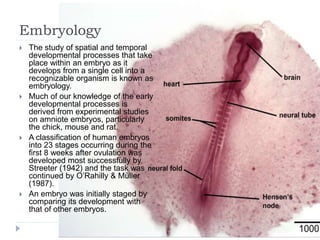
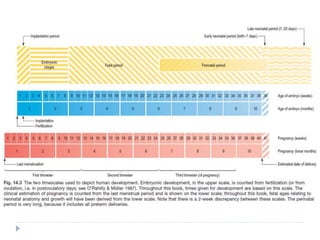
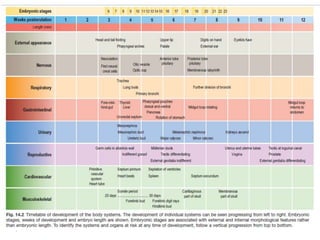
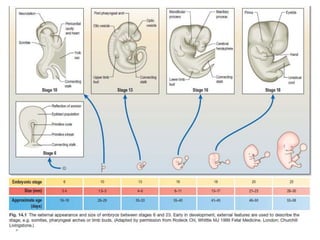
Blastulation refers to the process in early embryonic development where the zygote undergoes rapid cell divisions through cleavage to form a solid ball of cells called a morula. The morula then develops a fluid-filled cavity, forming a structure called a blastocyst composed of an inner cell mass and outer layer of trophoblast cells. The blastocyst undergoes further differentiation, with the inner cell mass forming the embryo and extraembryonic tissues such as the amnion, yolk sac, and allantois developing to support the growth and development of the embryo.