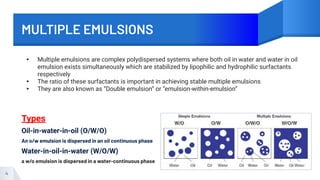
The document discusses emulsions, particularly microemulsions and multiple emulsions, detailing their definitions, types, advantages, and theories of formation. It emphasizes the stability issues related to emulsions, including flocculation, creaming, coalescence, breaking, and phase inversion, as well as methods for preserving emulsions and preventing microbial growth. Additionally, the rheological properties of emulsions are evaluated for various applications in drug delivery and stability studies.




![6
MONOMOLECULAR ADSORPTION THEORY
● Surfactants adsorb at the oil-water interface and form a
monomolecular film
This film rapidly envelopes the droplets
● They are very compact, elastic, flexible, strong and cannot be
easily broken
● For getting better stable emulsions combination of surfactants
[surfactant blend] are used rather than a single one
● The surfactant blend consists of both water soluble and oil
soluble surfactants in order to approach the interface from
aqueous and oil phase sides
● At interface the surfactant blend interact to form a complex and
condense a monomolecular film
● Ex: A combination of Sodium cetyl sulfate (hydrophilic) and
Cholesterol (lipophilic) forms a close packed complex film at
the interface that produces an excellent emulsion
● Surfactant blend also produces poor quality emulsions if the
interaction between them is not strong enough at the interface](https://image.slidesharecdn.com/coarsedispersions-emulsions-220130063211/85/Coarse-dispersions-emulsions-6-320.jpg)












