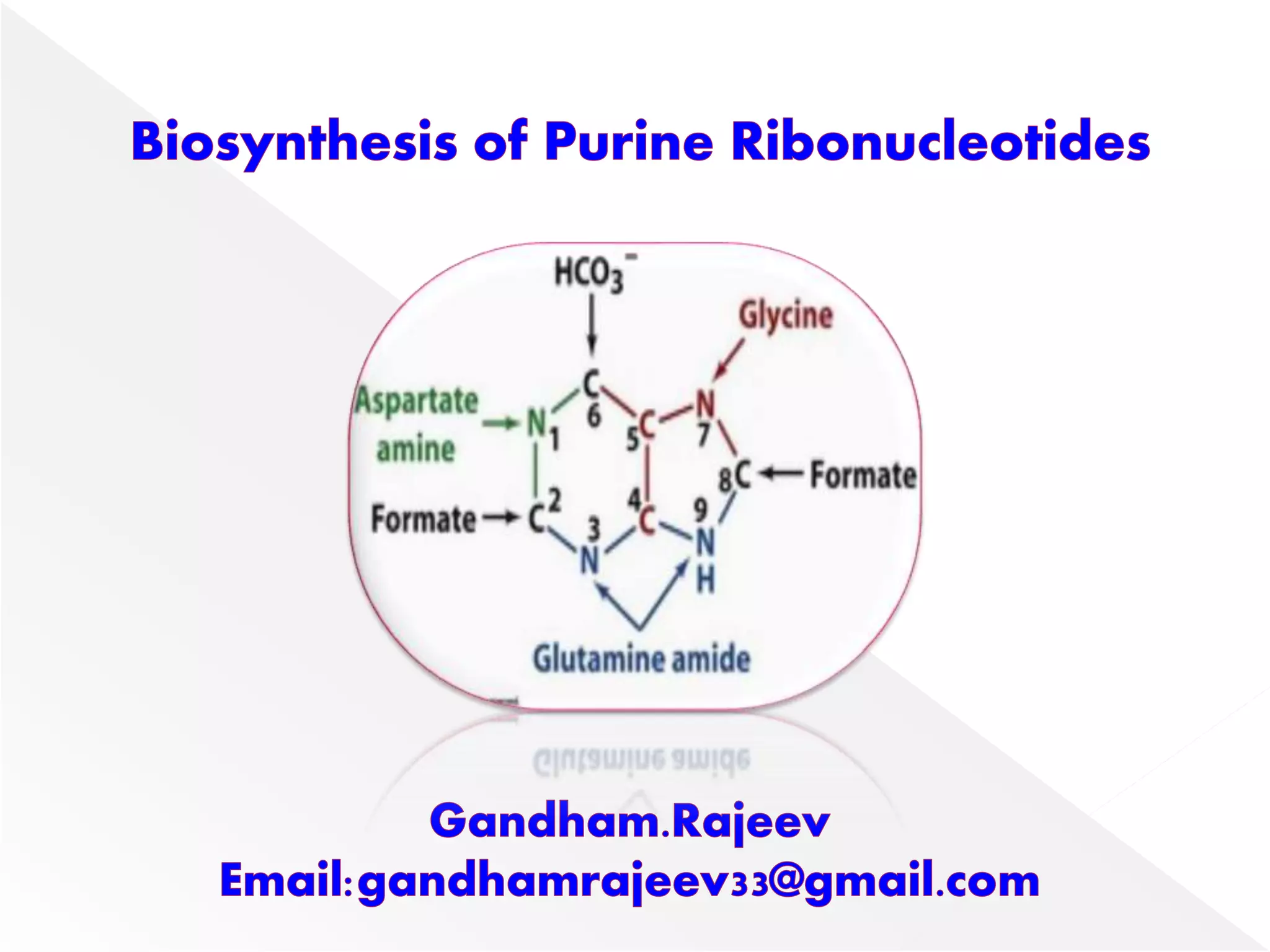
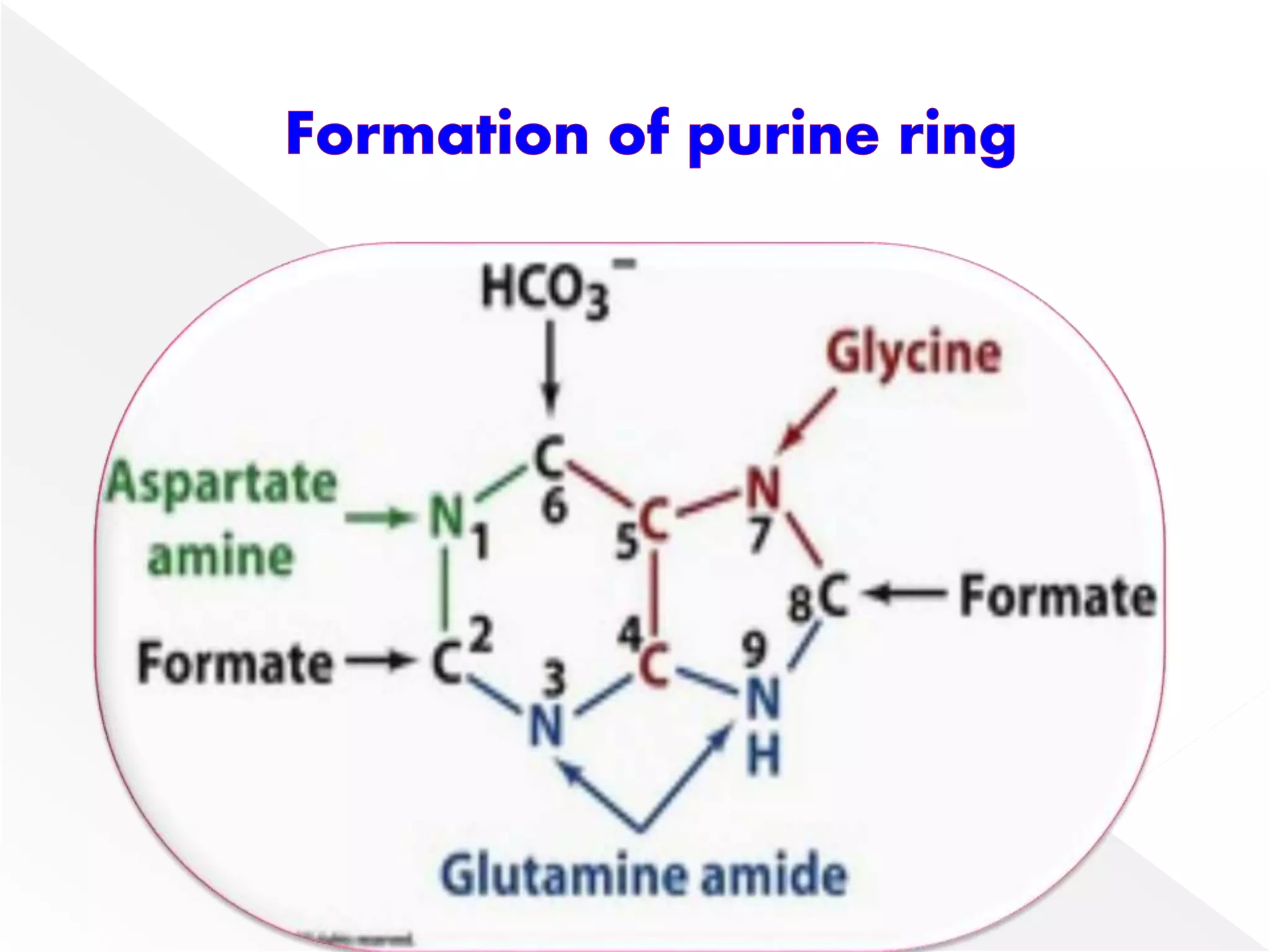
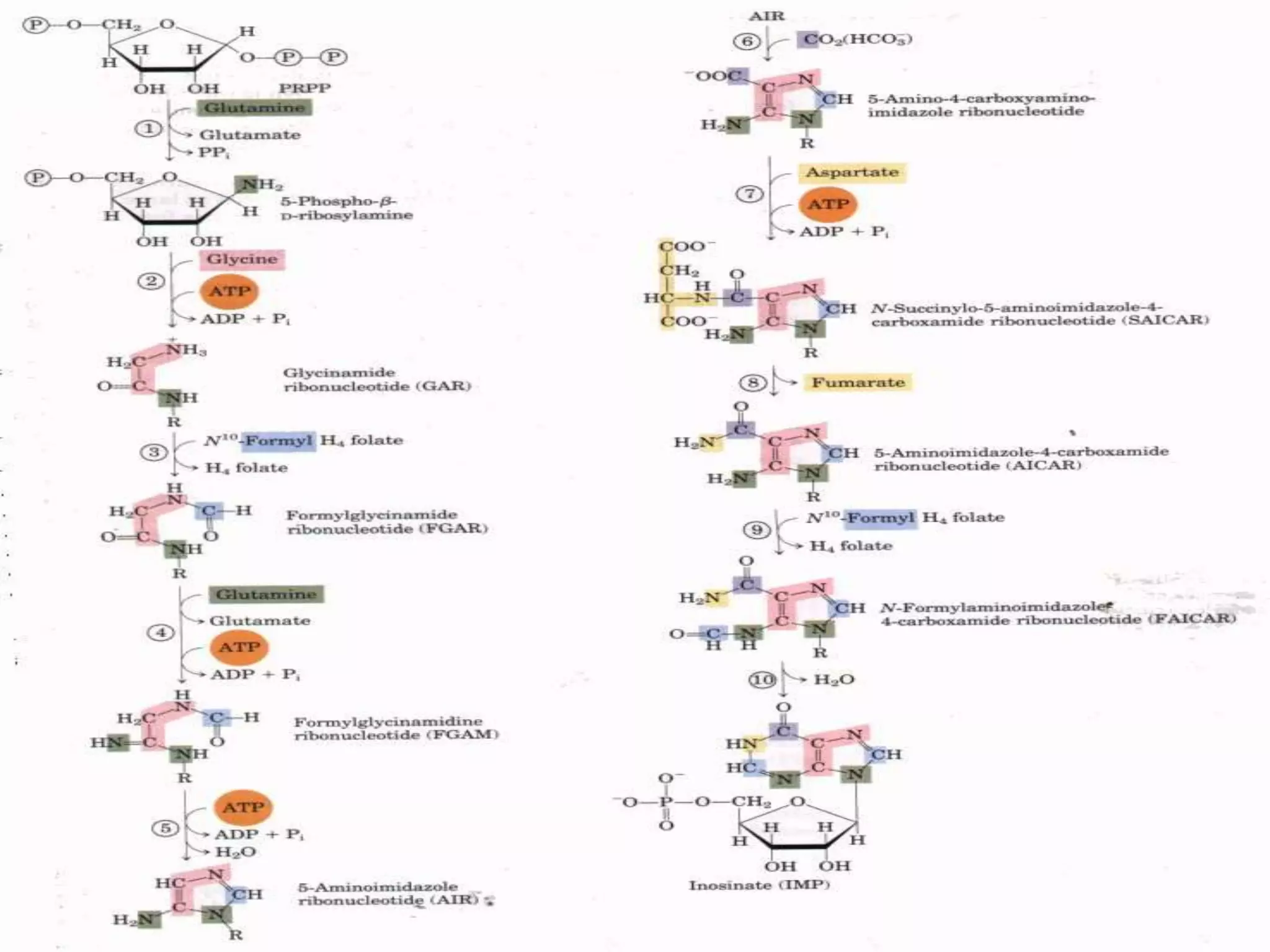
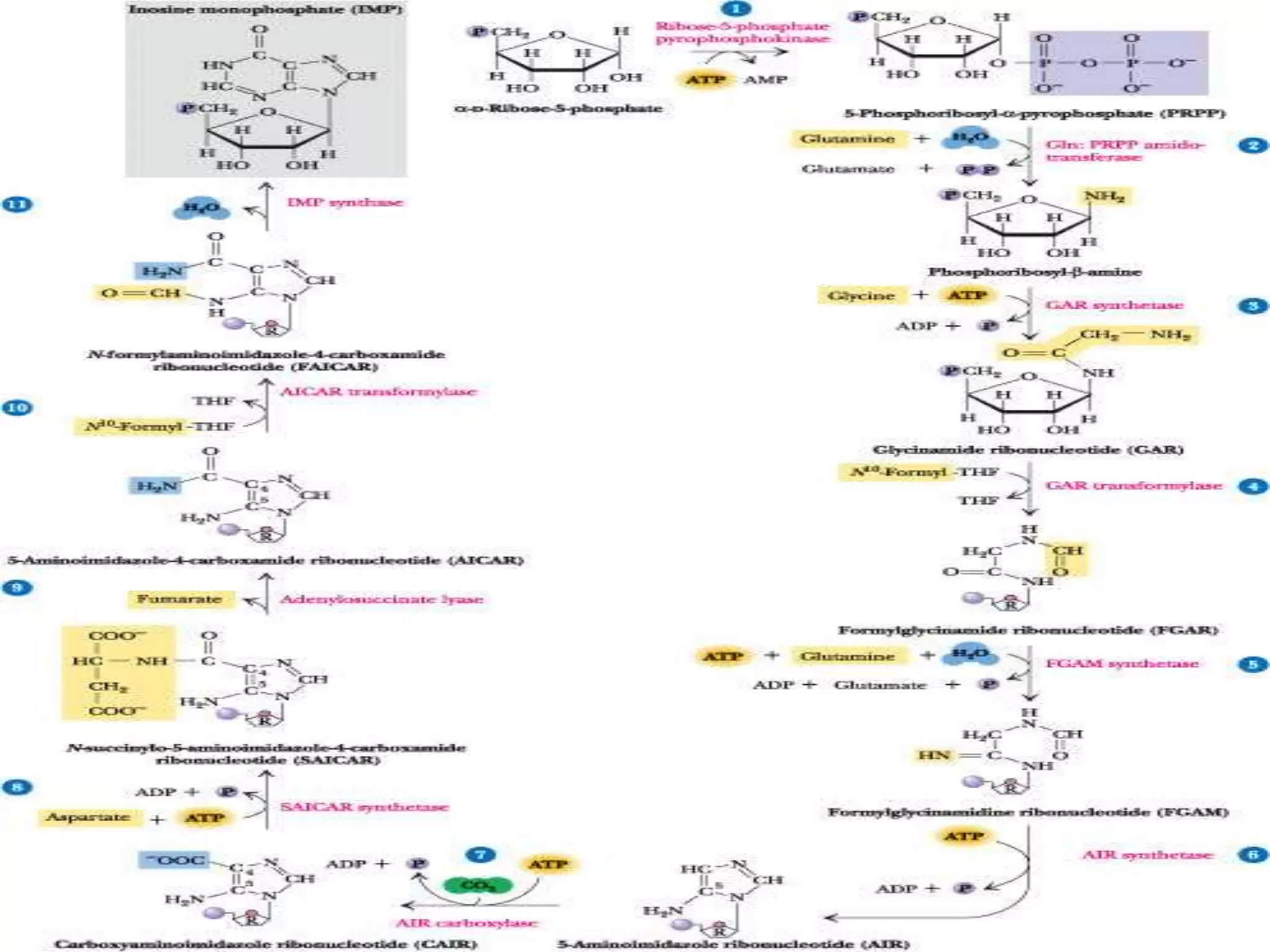
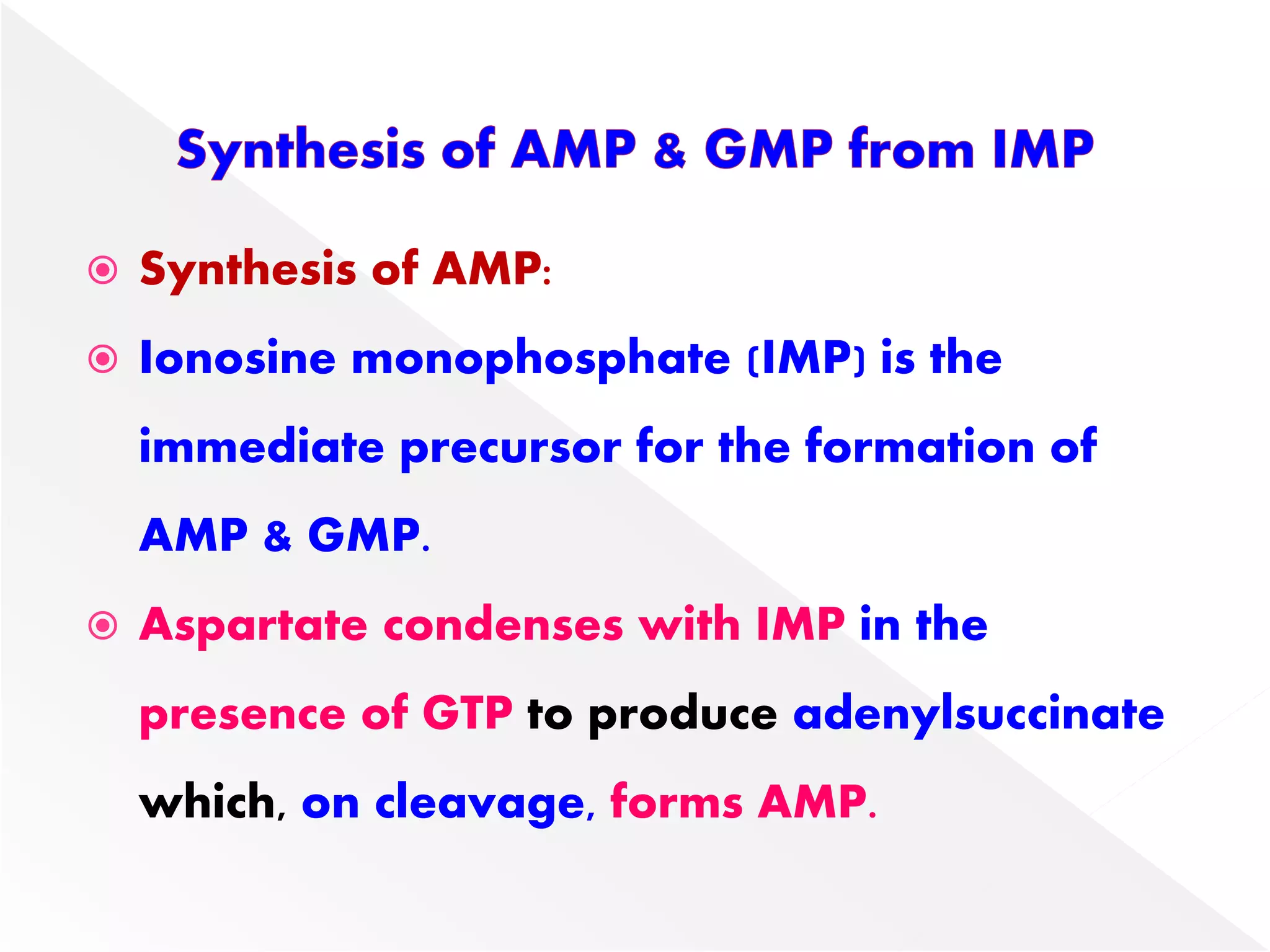
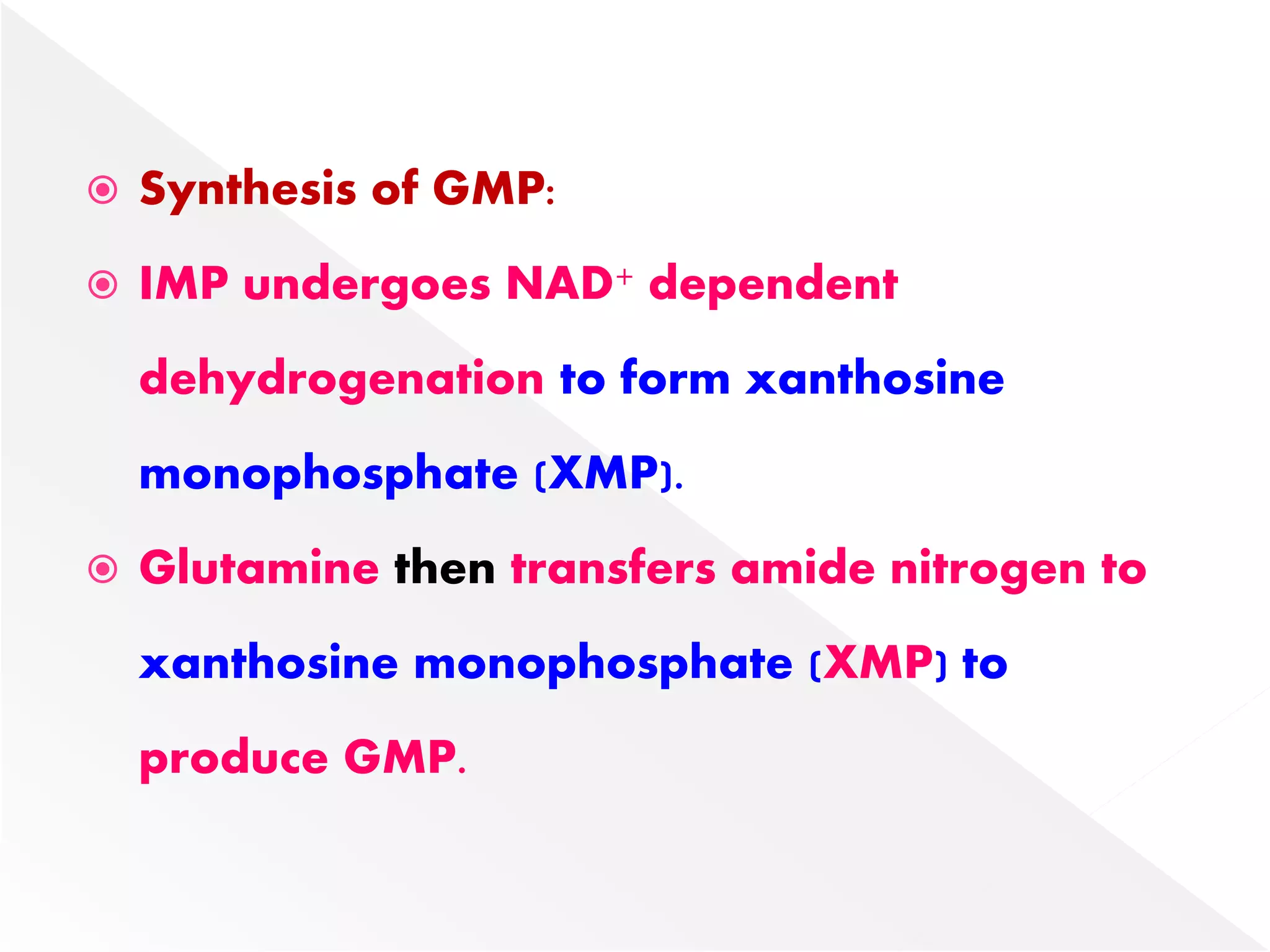
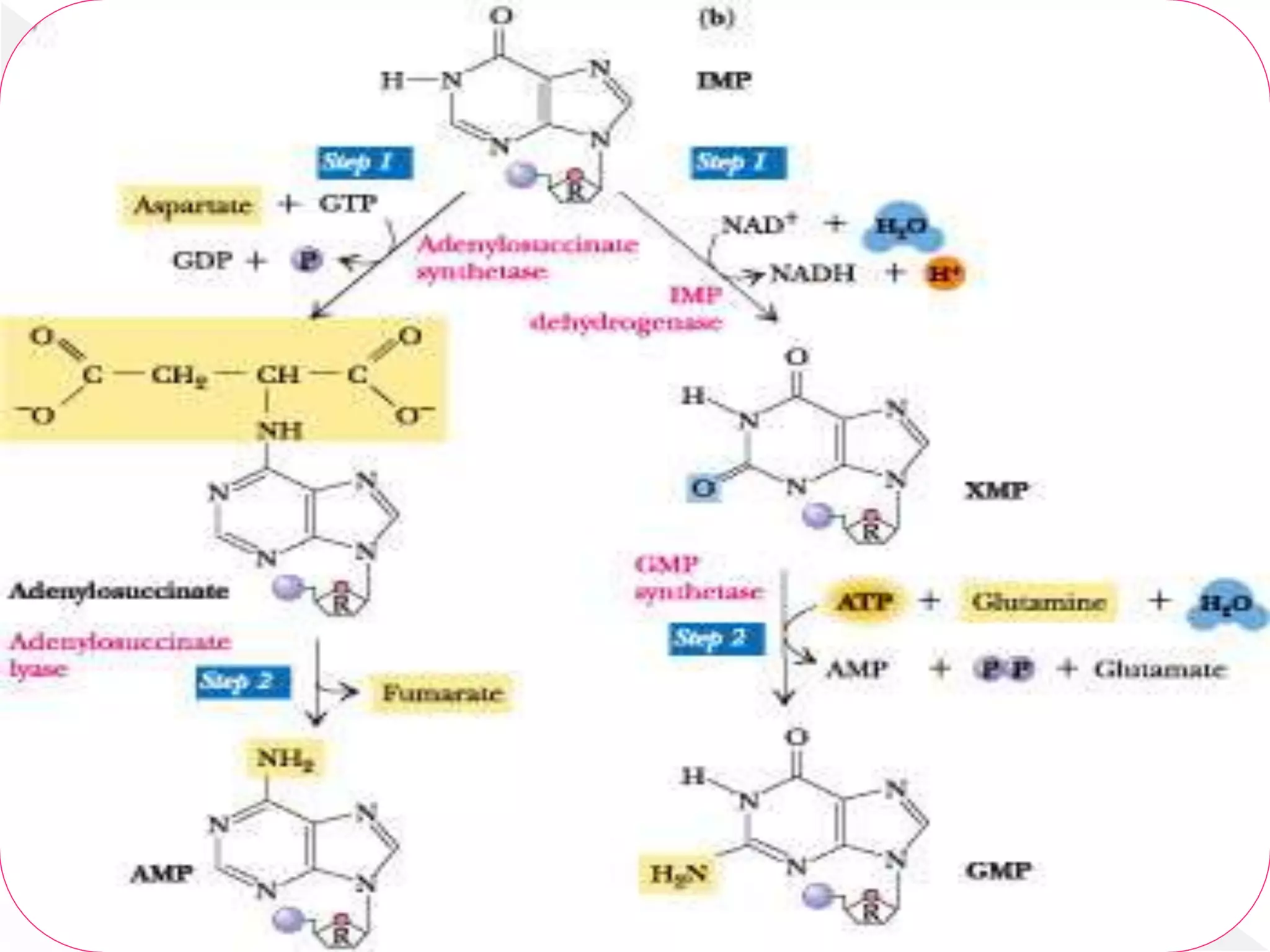
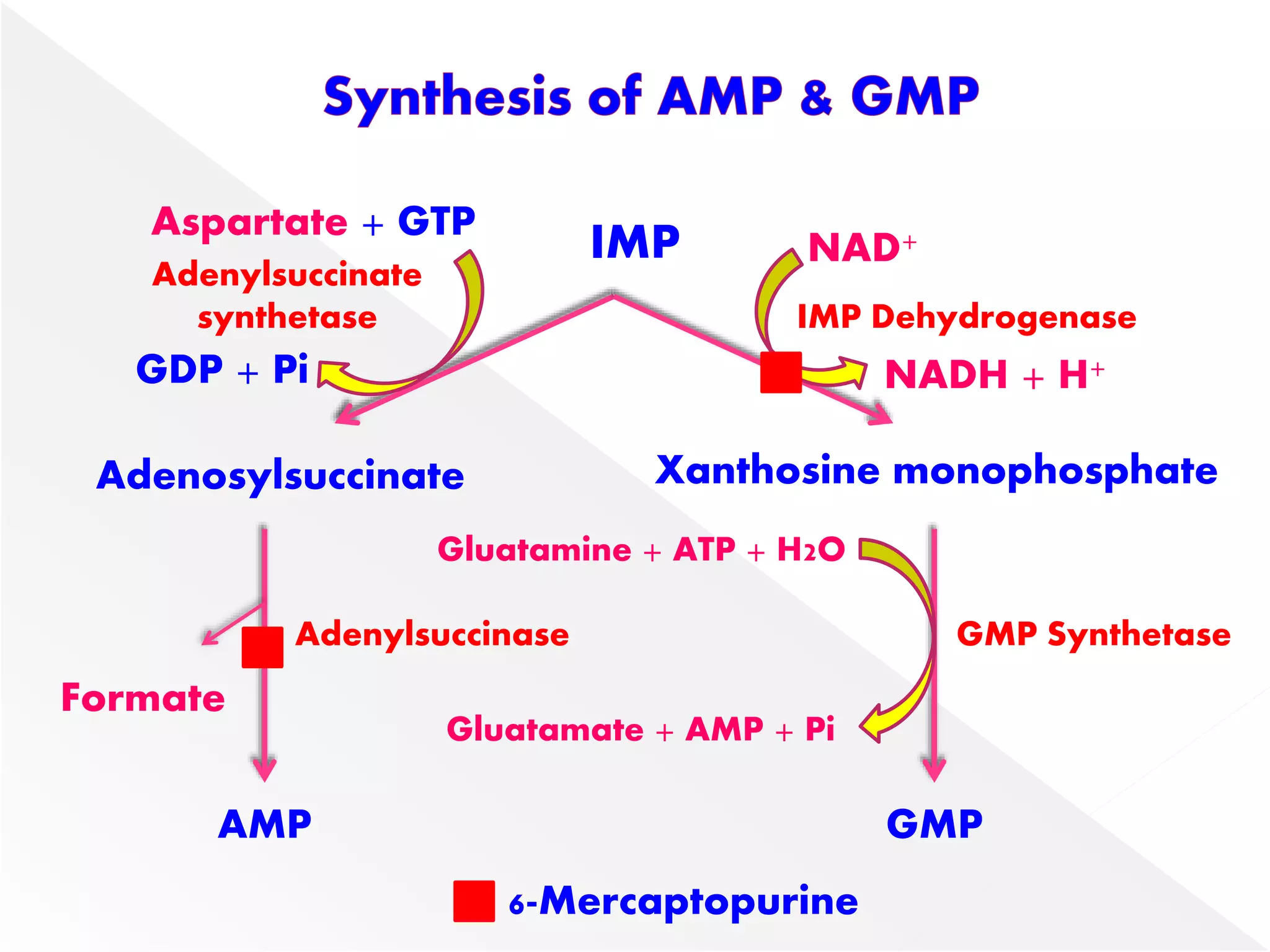
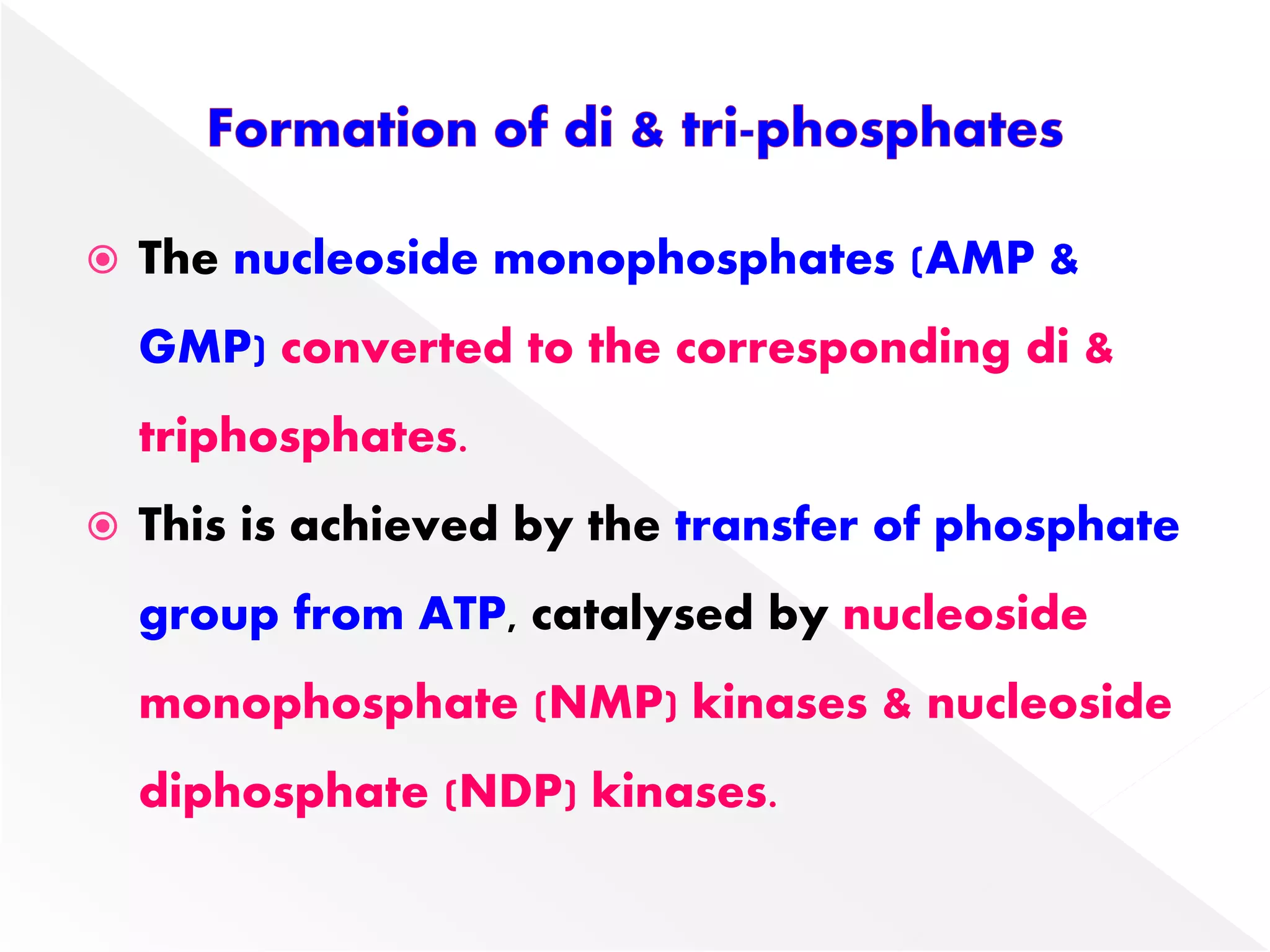
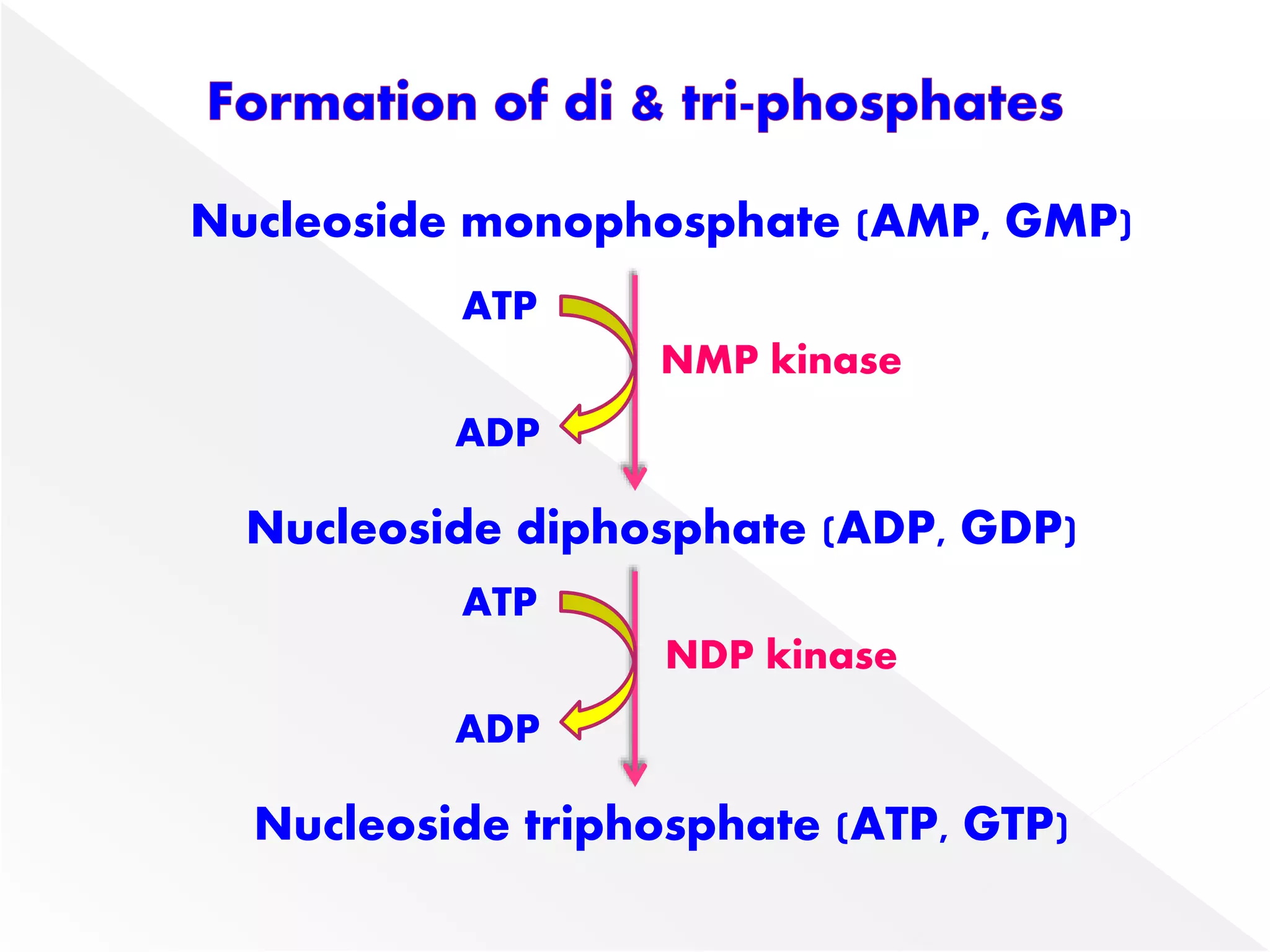
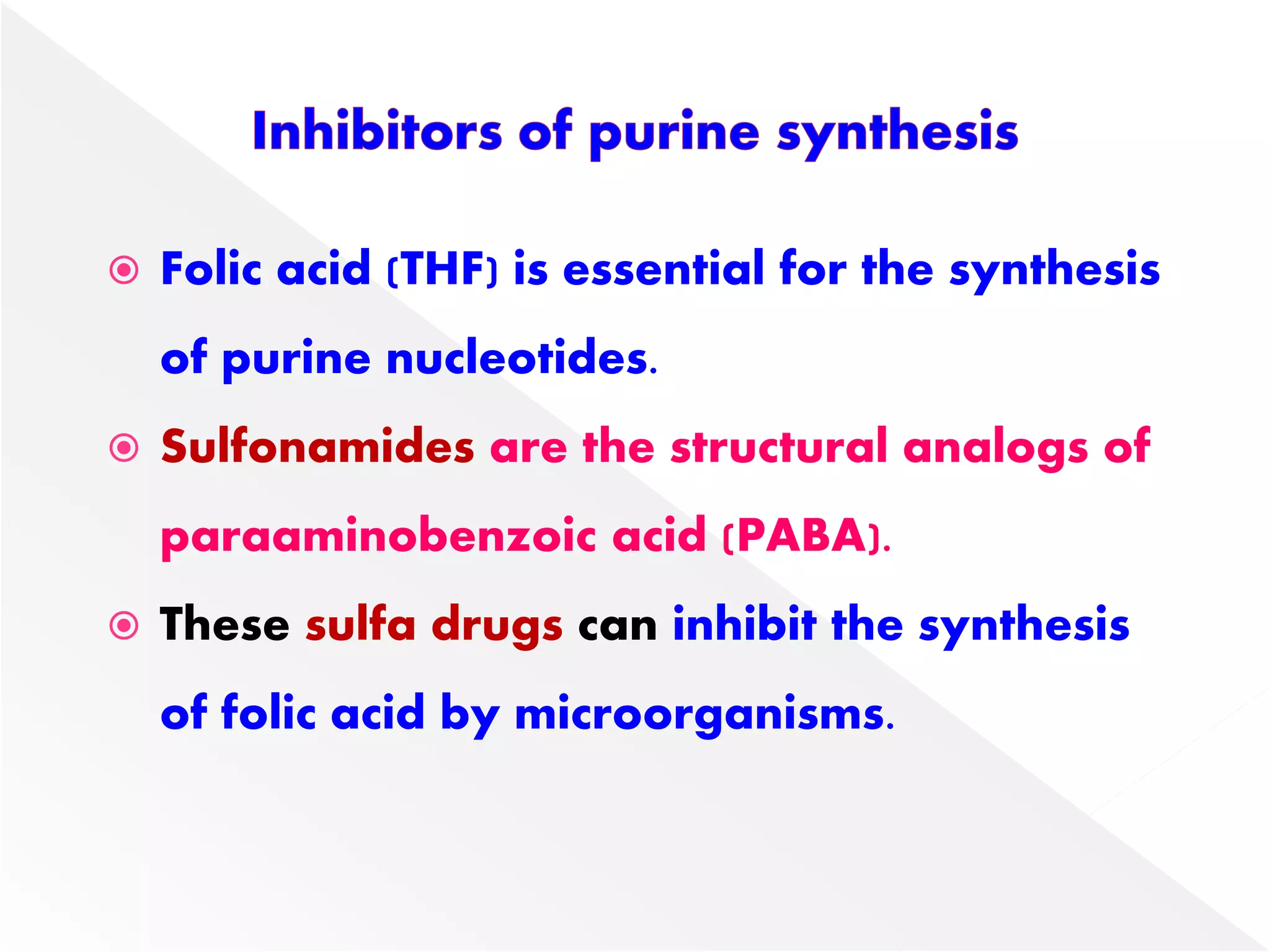
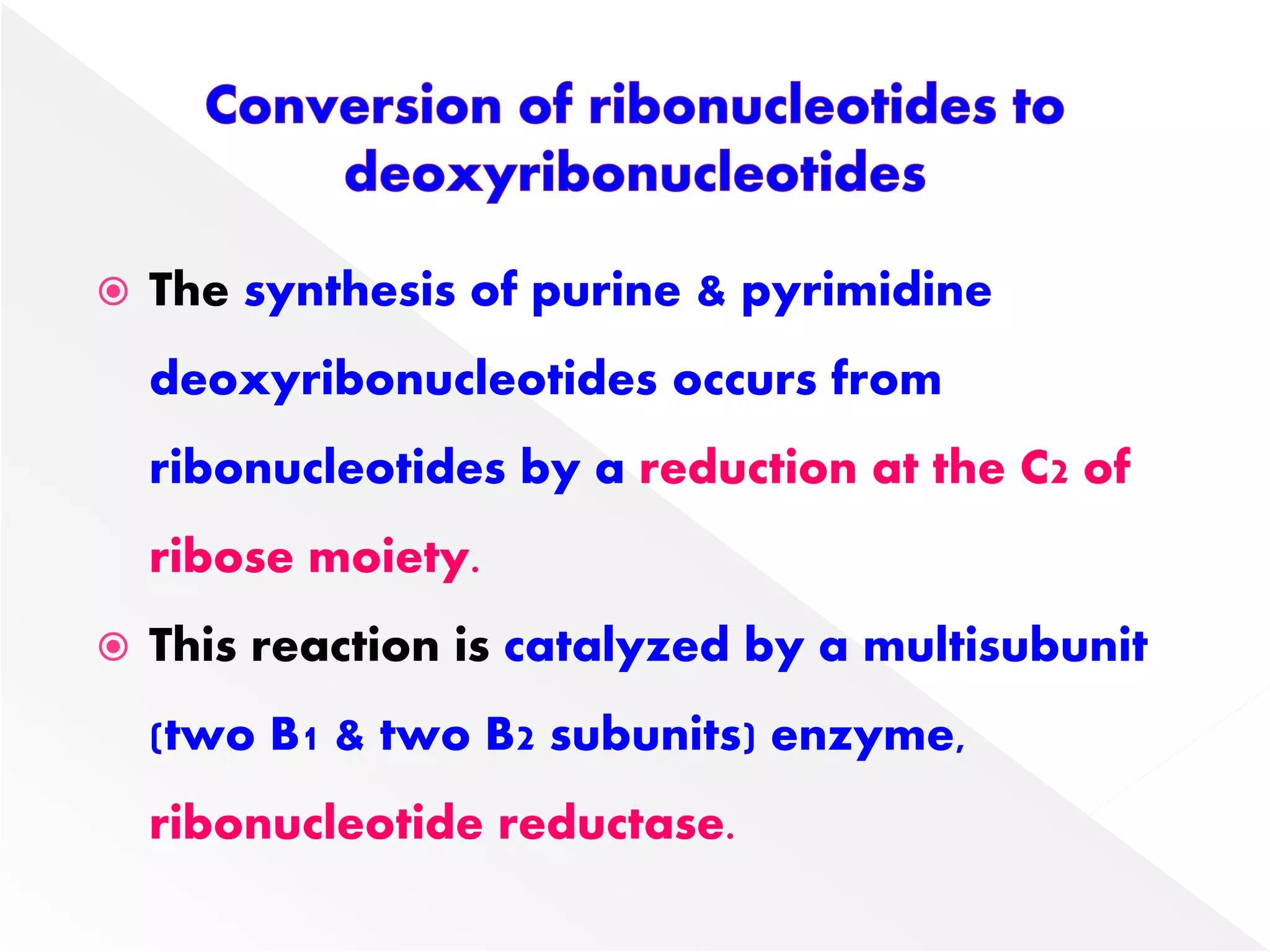
1. The document summarizes purine nucleotide synthesis, which involves multiple enzymatic reactions using substrates like aspartate, glutamine, glycine, and CO2 to build the purine ring structure on ribose 5-phosphate.
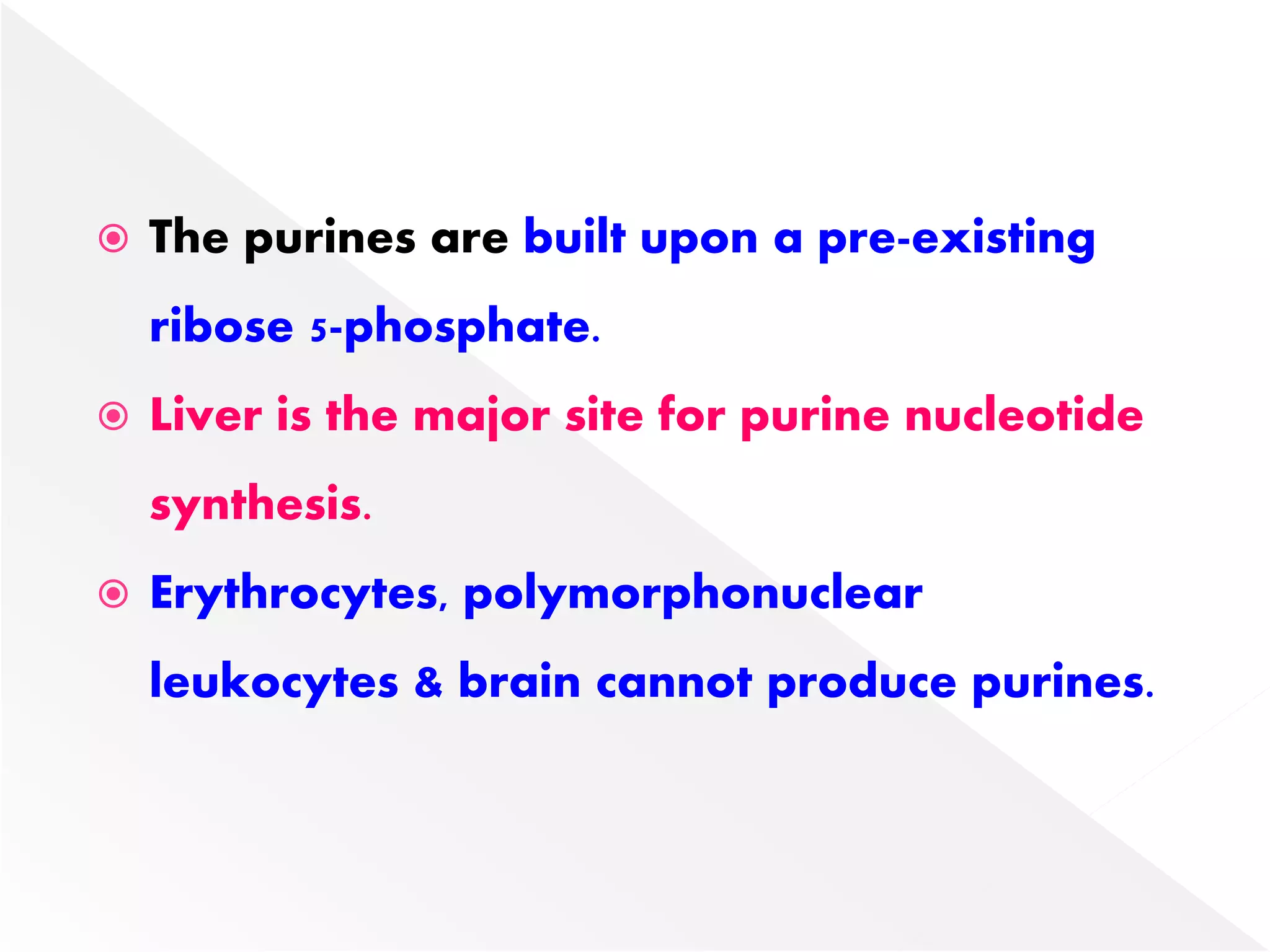
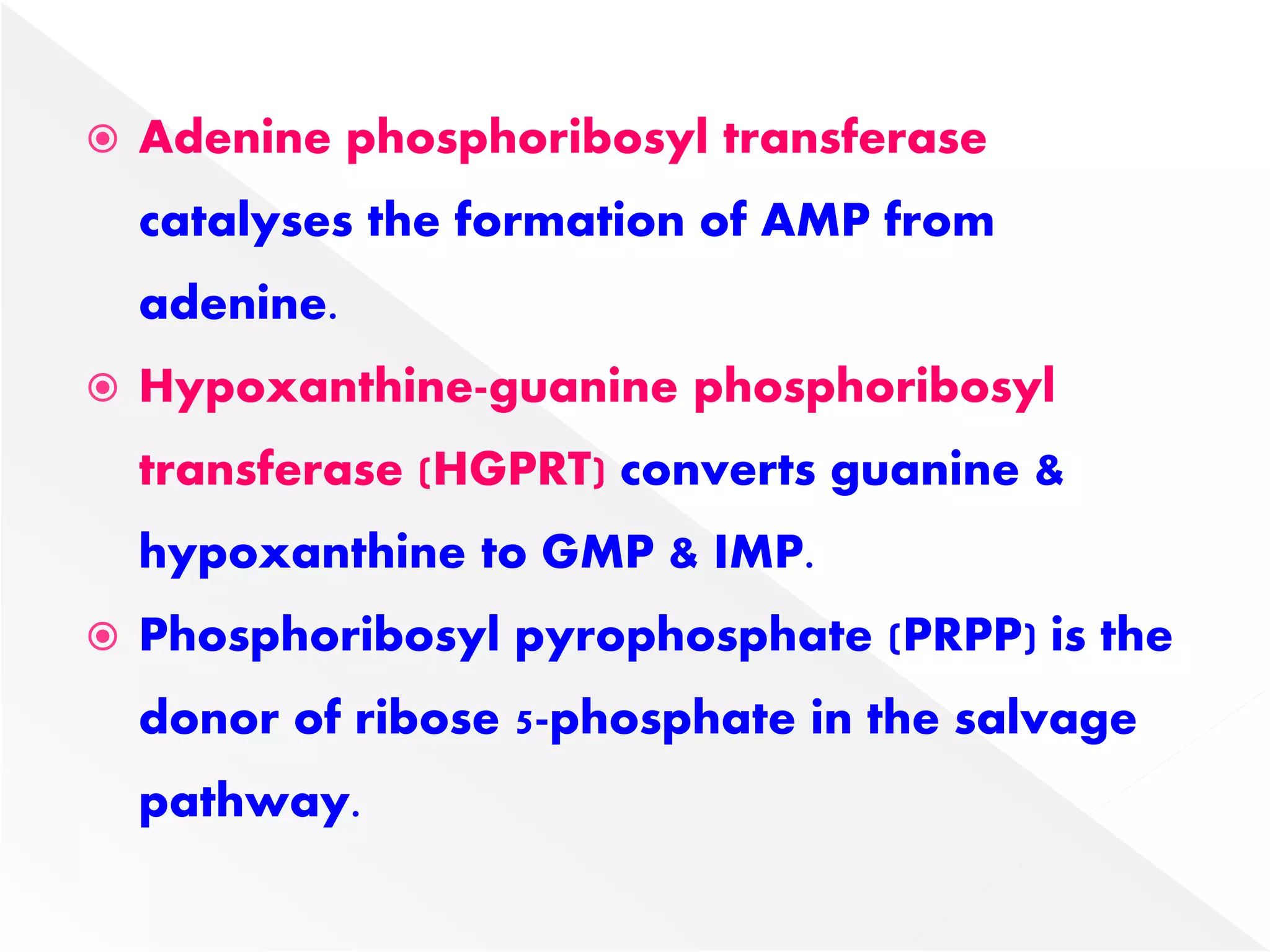
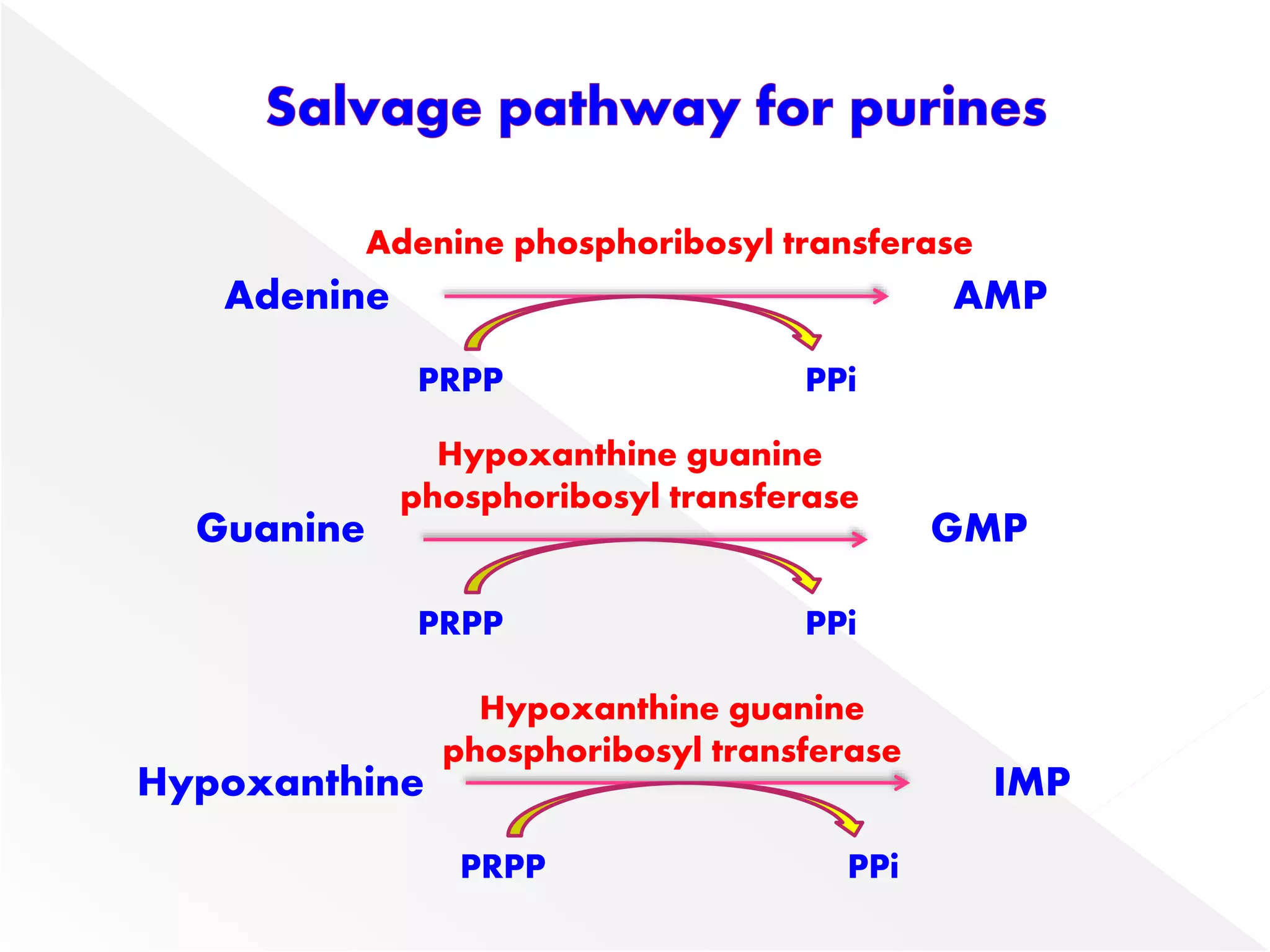
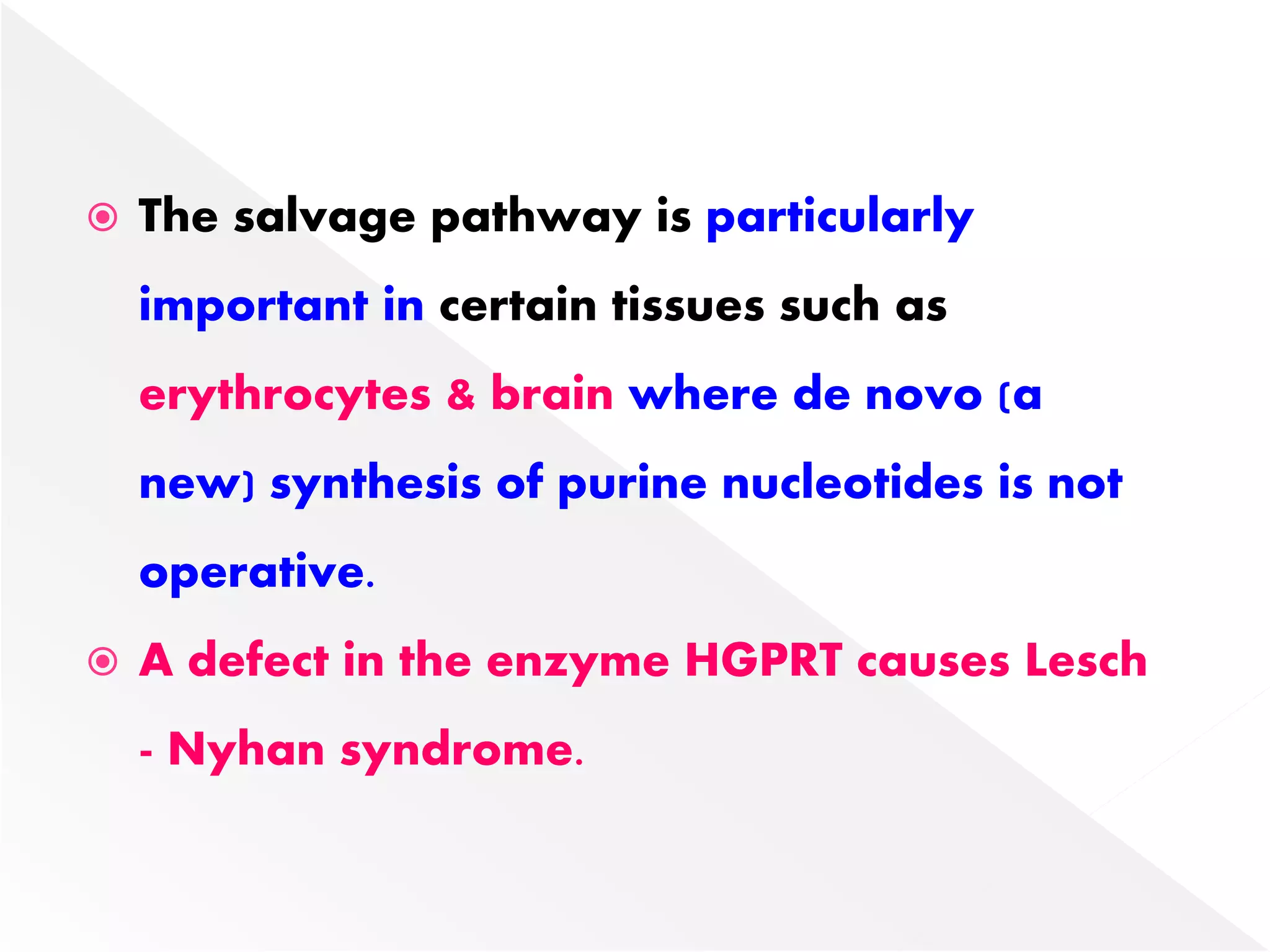
2. Liver is the major site of de novo purine synthesis, while erythrocytes and brain must salvage purines due to their inability to synthesize them.
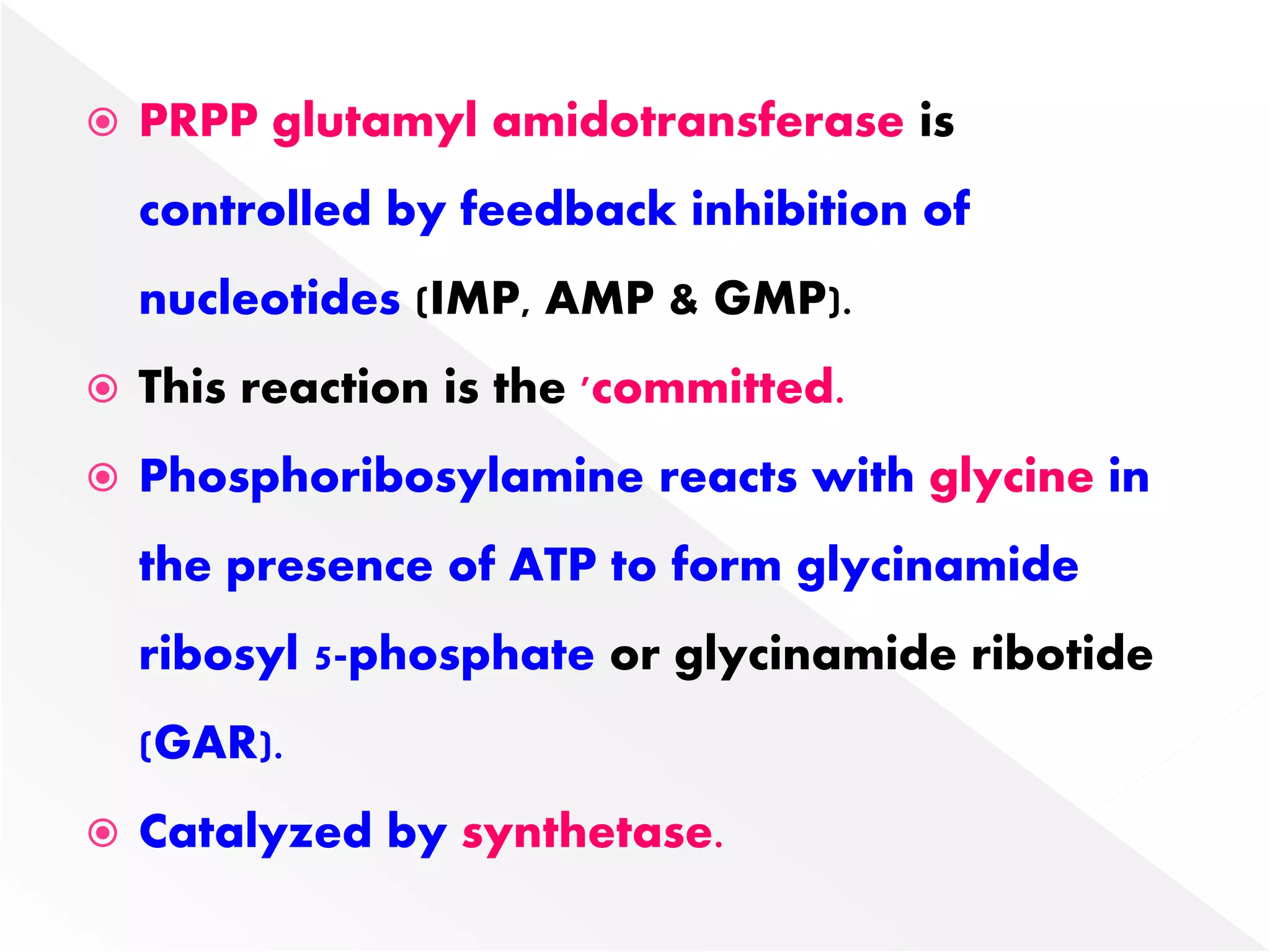
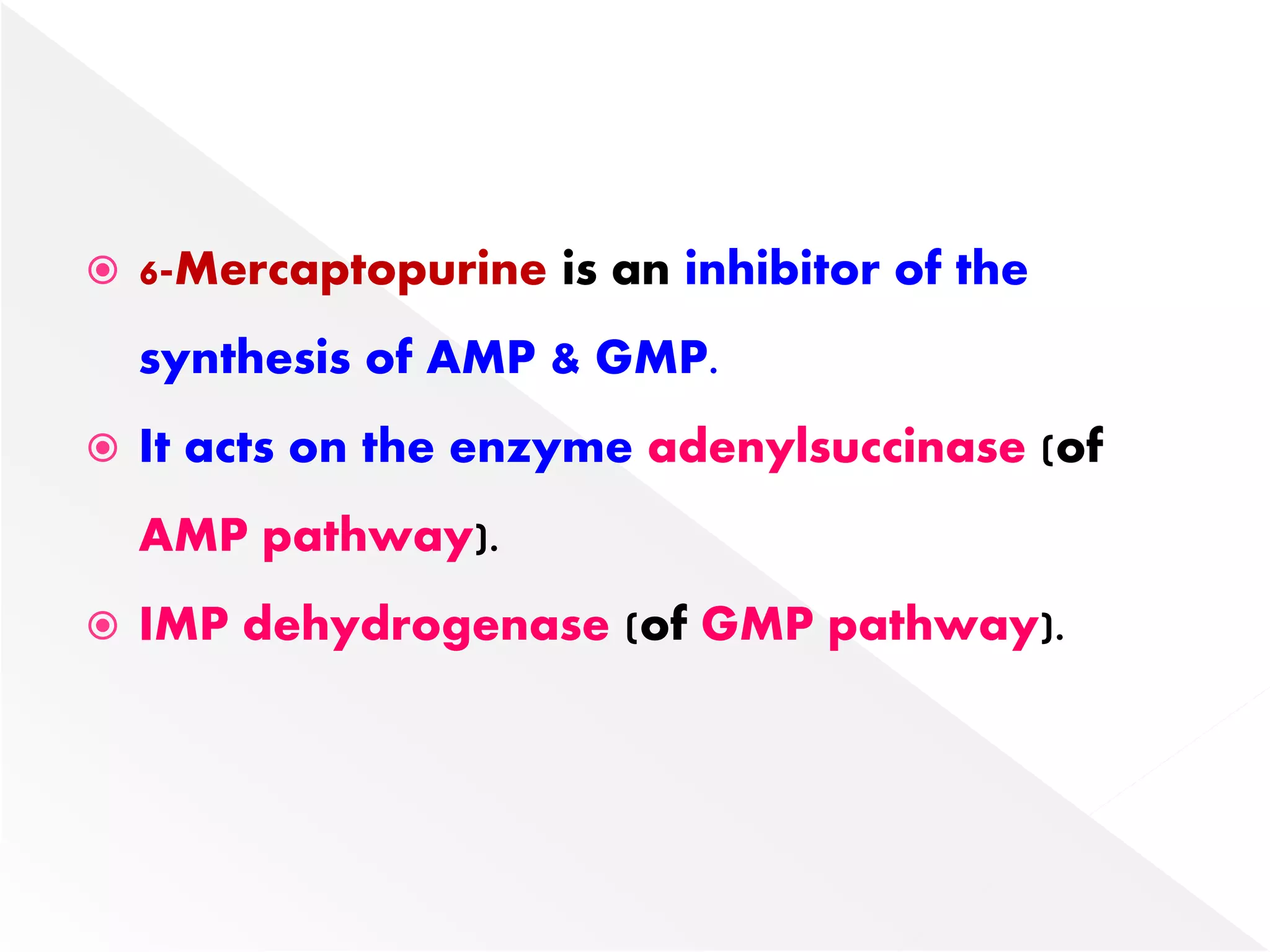
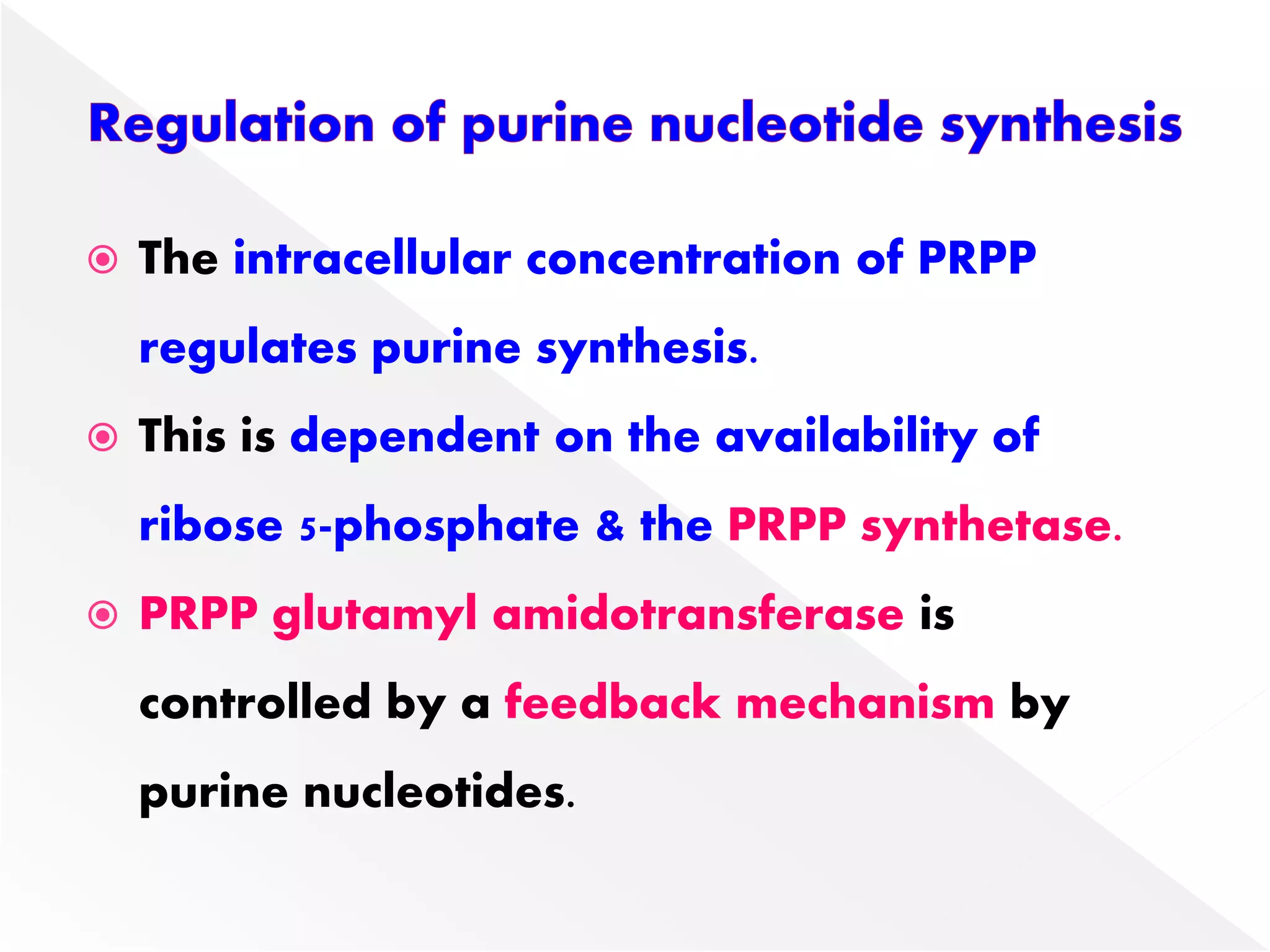
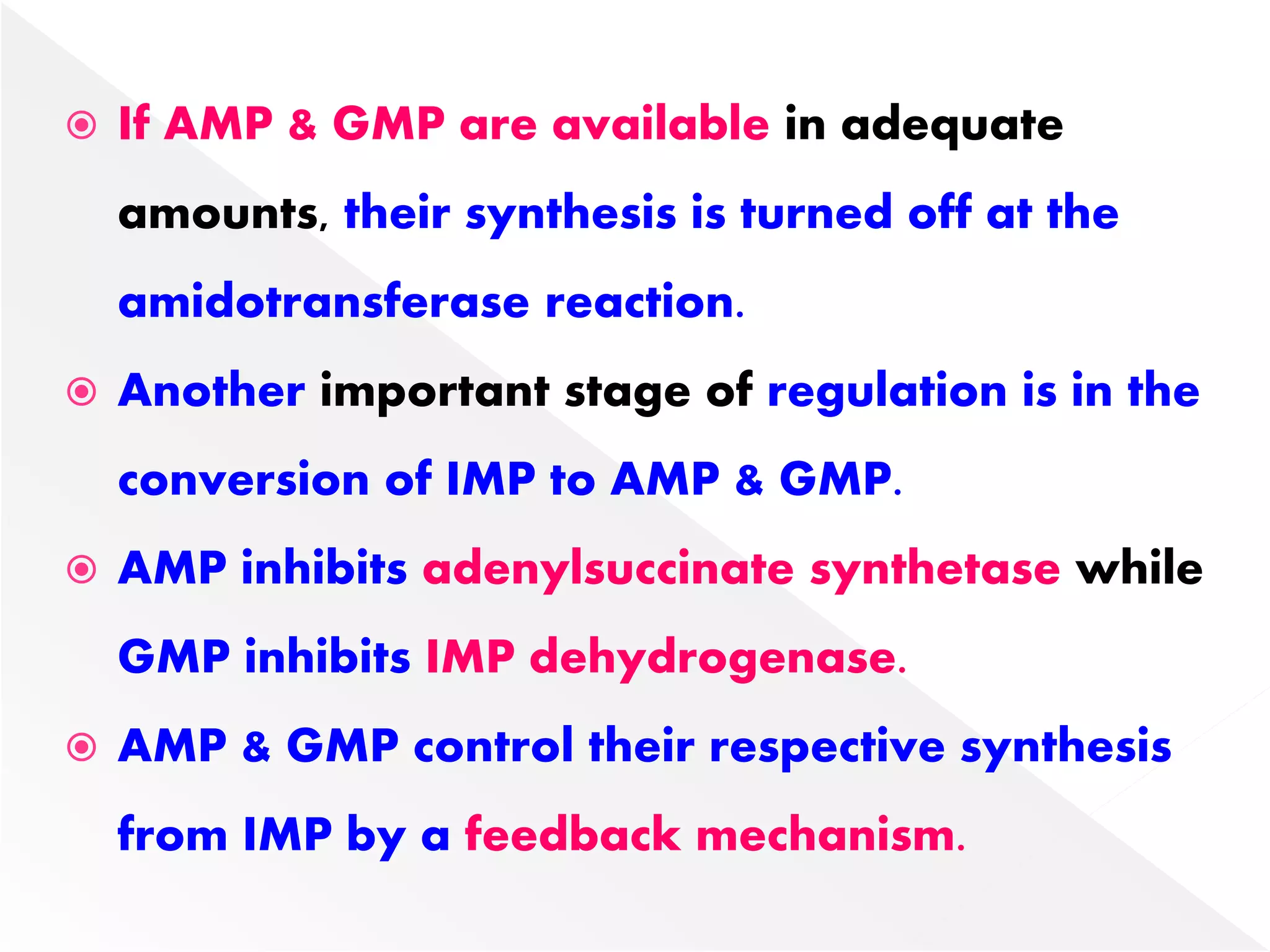
3. Feedback inhibition regulates purine synthesis at committed steps, and analogs like 6-mercaptopurine can inhibit pathways leading to AMP and GMP formation.