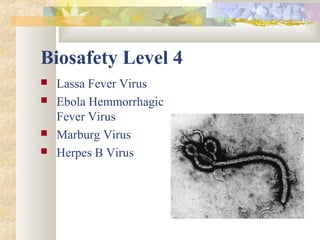
The document discusses basic principles of biosafety. It begins by defining biosafety as safety from exposure to infectious agents. It then discusses biosafety levels 1-4 which provide increasing levels of containment for biological hazards. It also discusses important biosafety concepts like standard microbiological practices, safety equipment, facility design and construction that help prevent exposure to biological hazards in research and clinical settings.