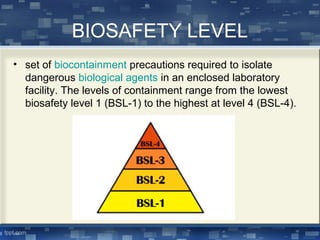
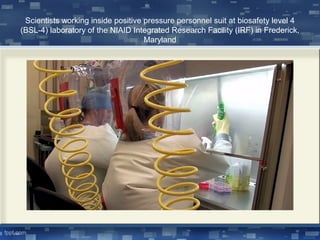
Biosafety refers to ensuring safety when working with biological organisms. This document discusses biosafety concepts and issues including containment levels, biosafety cabinets, and risk assessment. The four biosafety levels range from level 1 posing minimal risk to level 4 posing high individual risk without vaccines or treatments. Biosafety cabinets are used to protect workers and the environment, with class I protecting environment, class II protecting samples and environment, and class III providing maximum protection in BSL-4 labs. Risk assessment considers an organism's pathogenicity, virulence, proliferation ability, and transmission route. Guidelines for recombinant DNA research emphasize risk-based containment and avoiding unnecessary regulation.