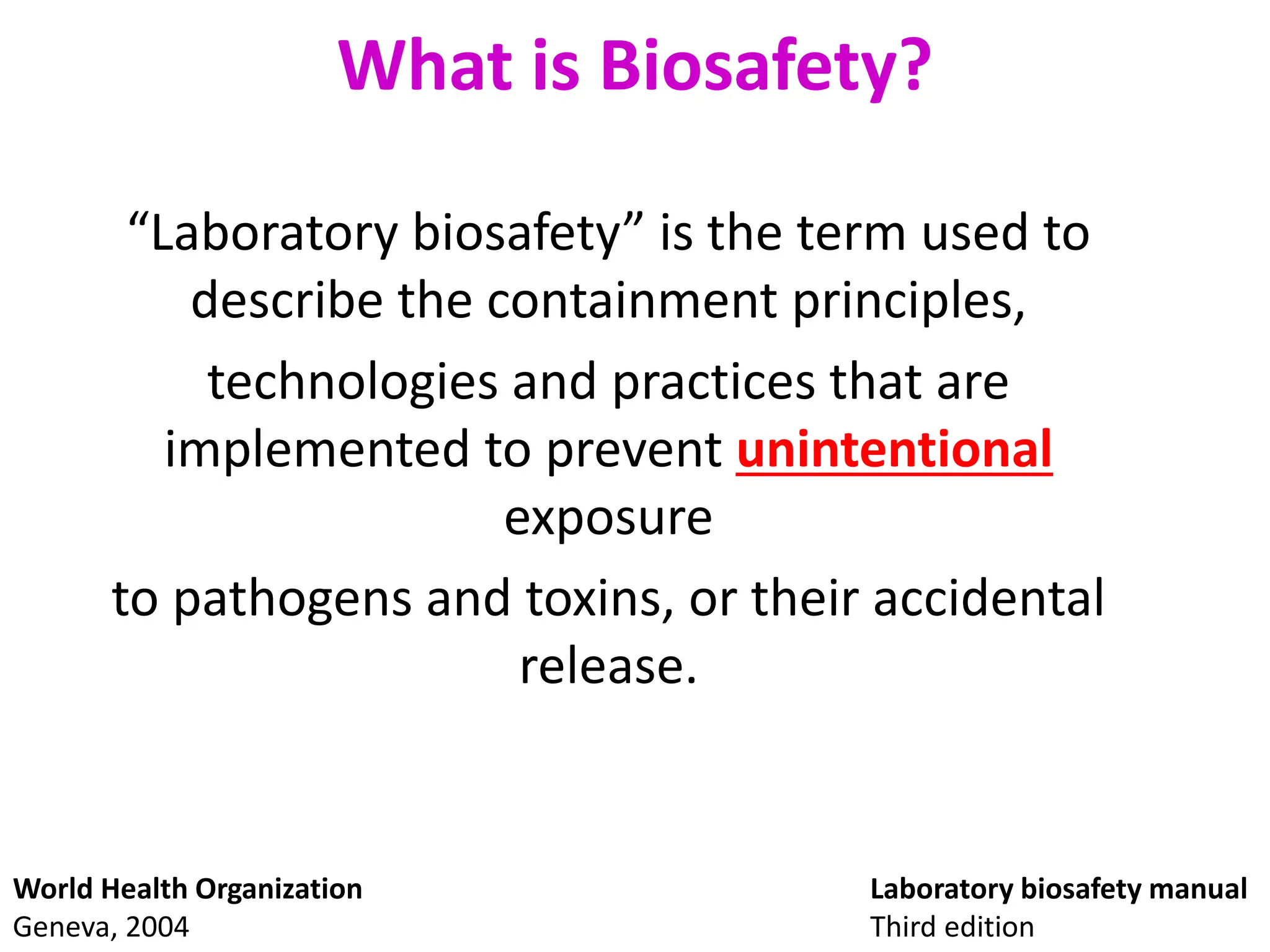
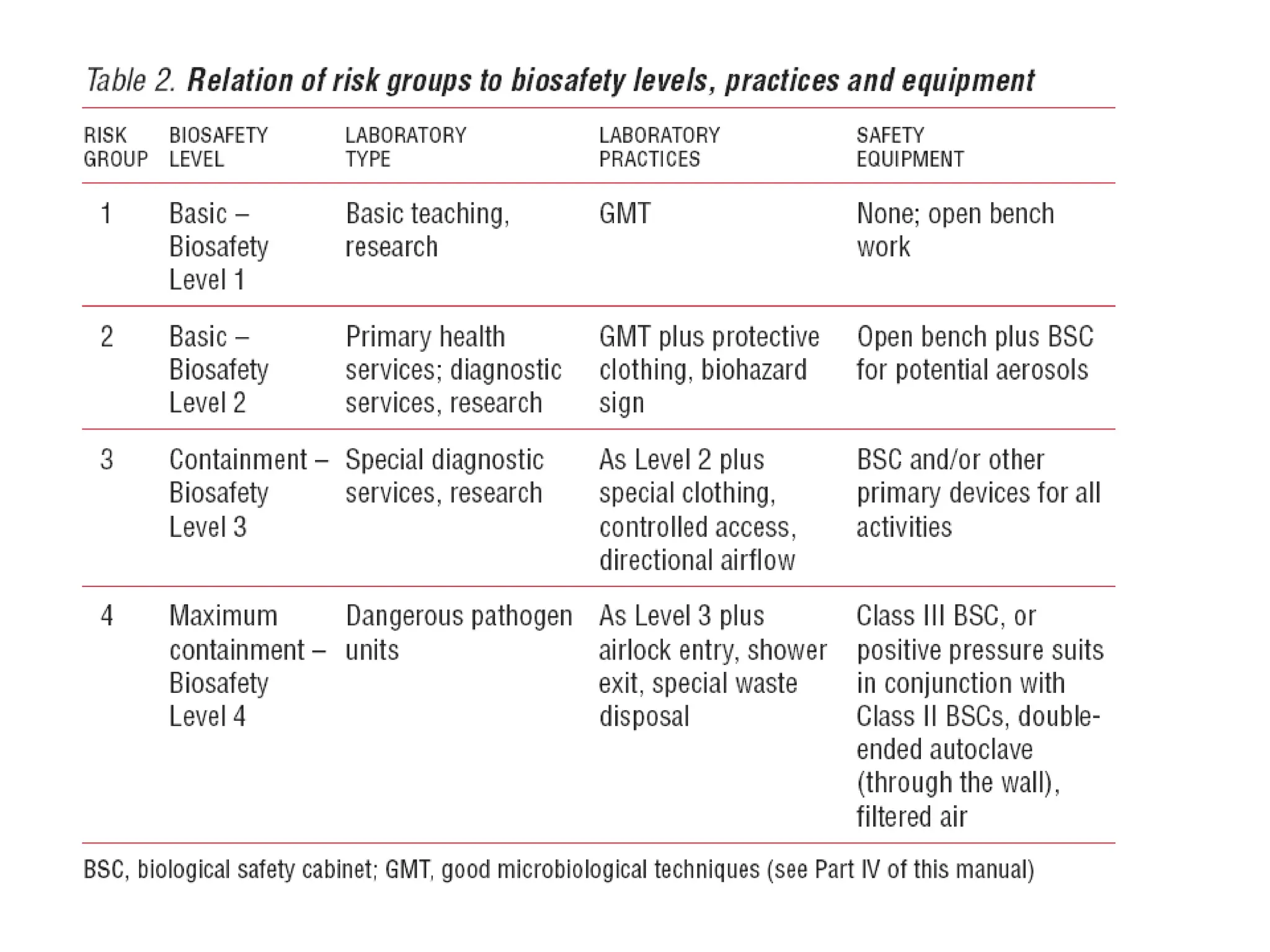
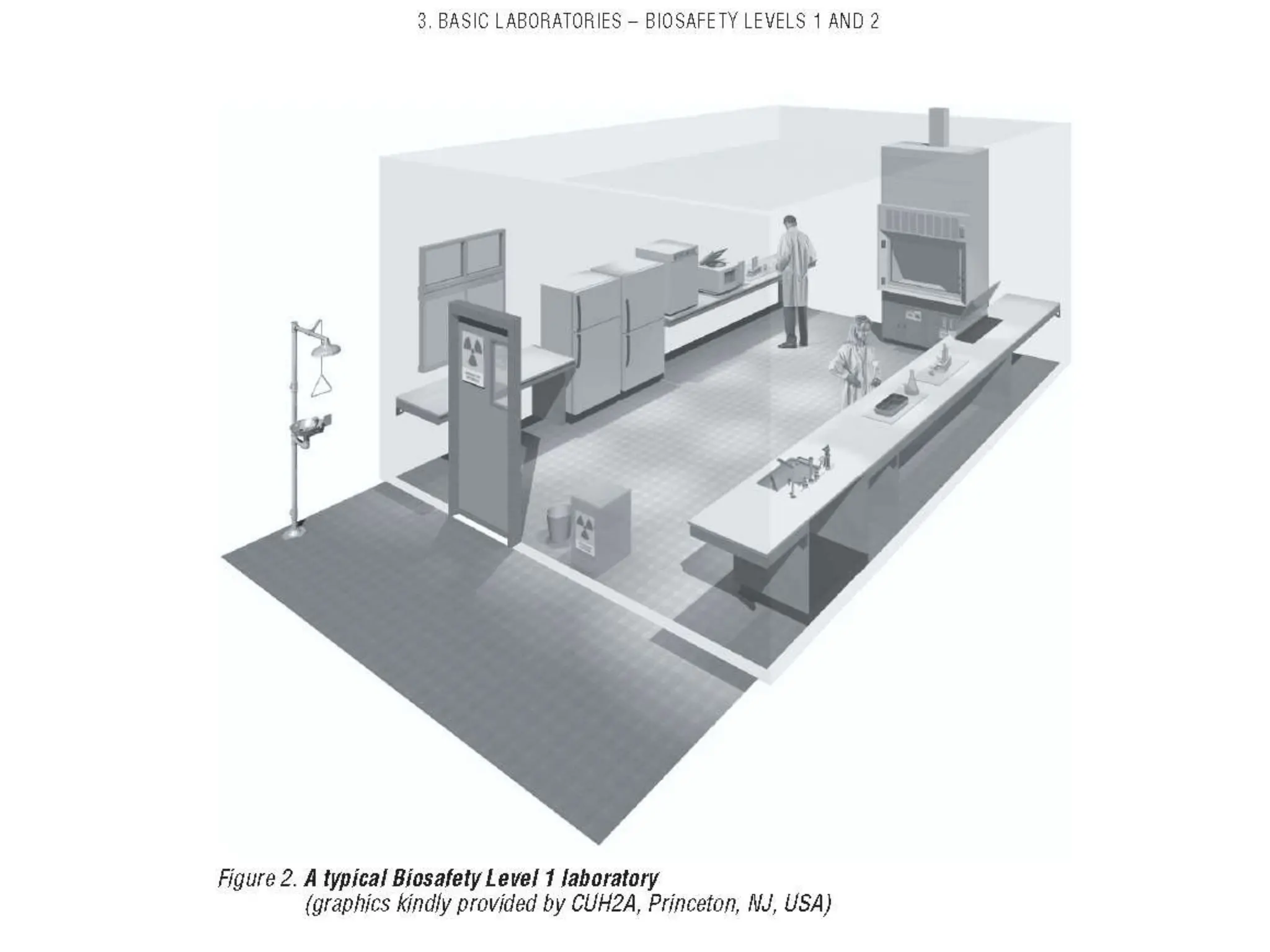
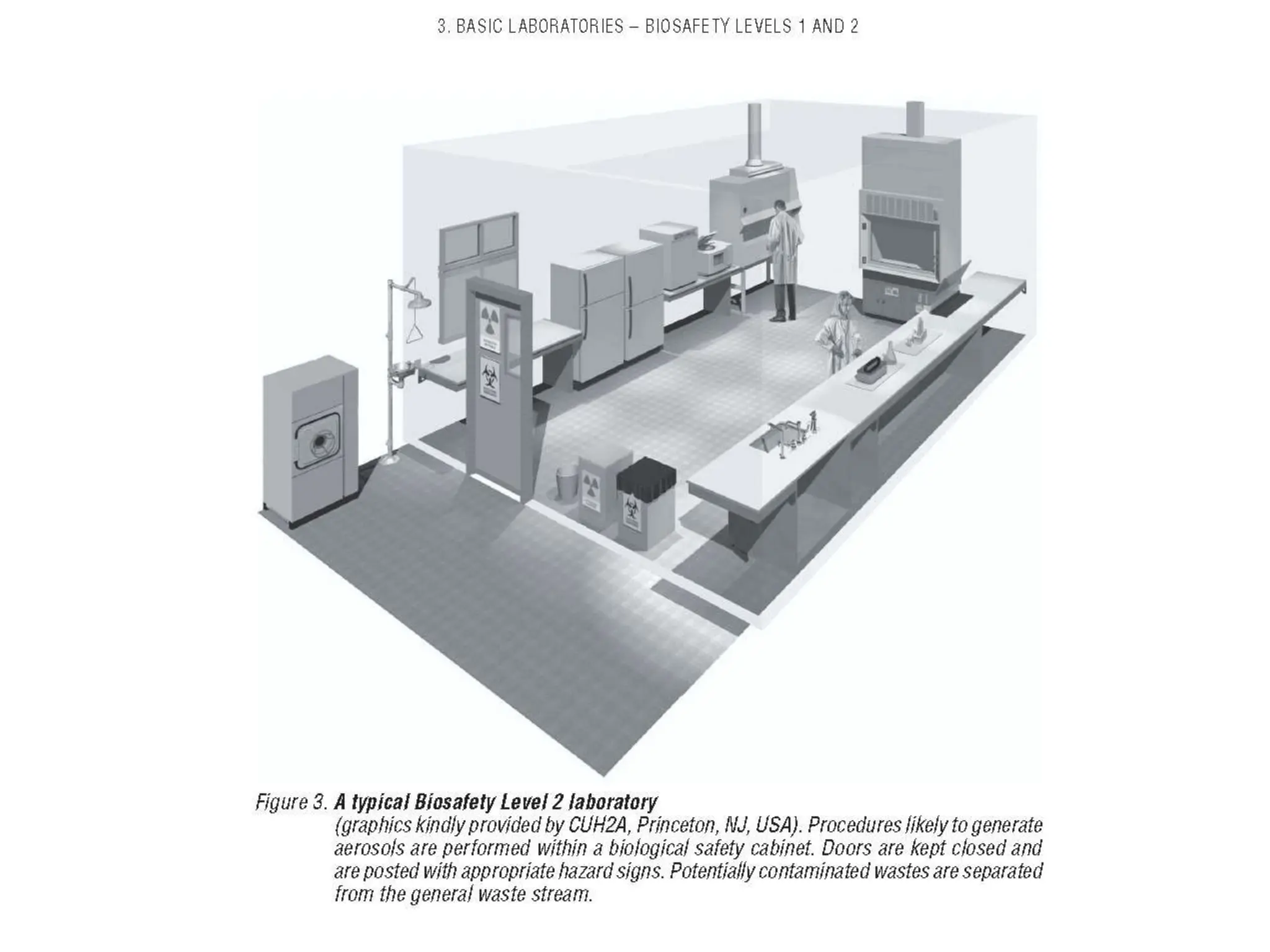
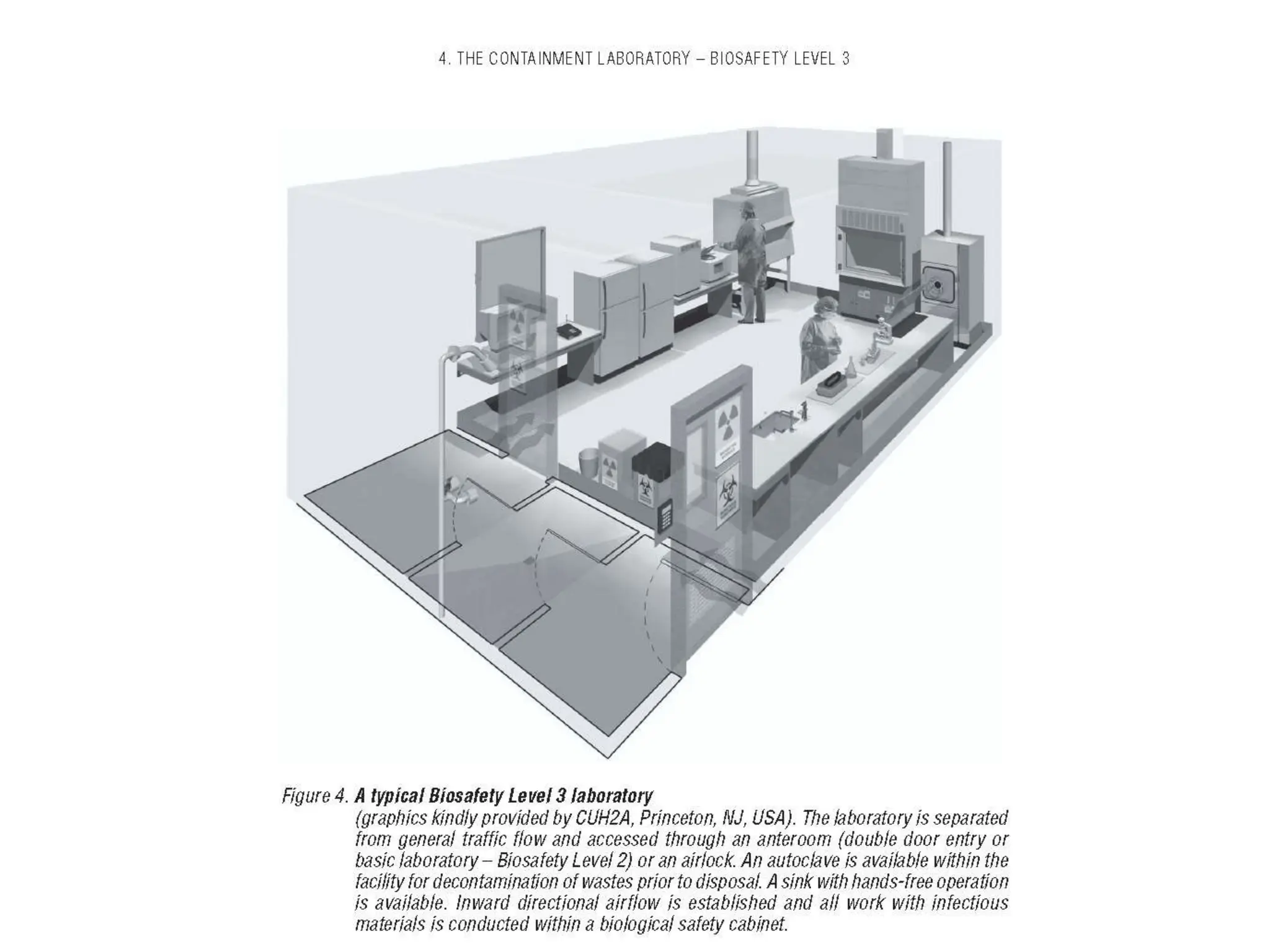
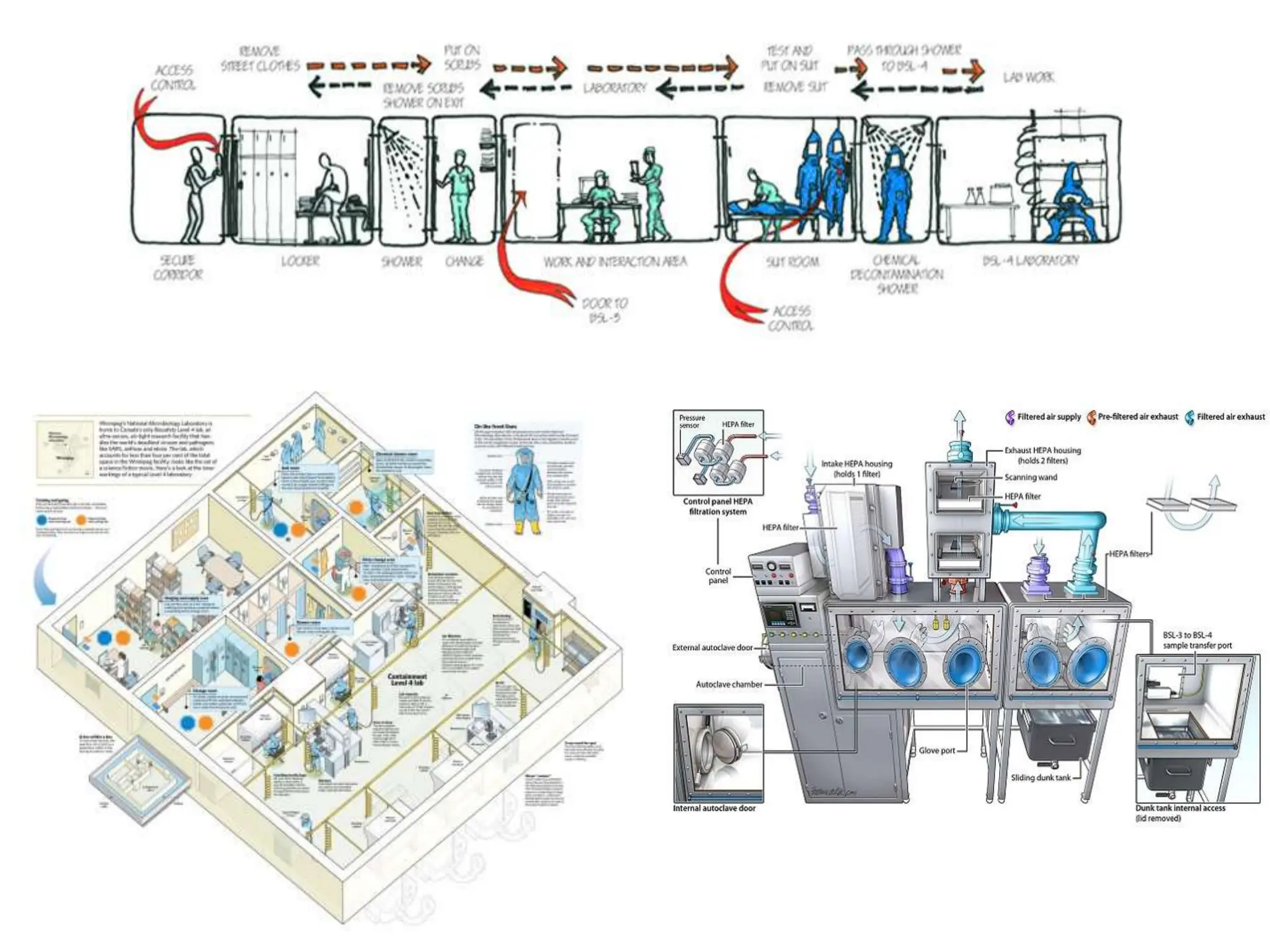
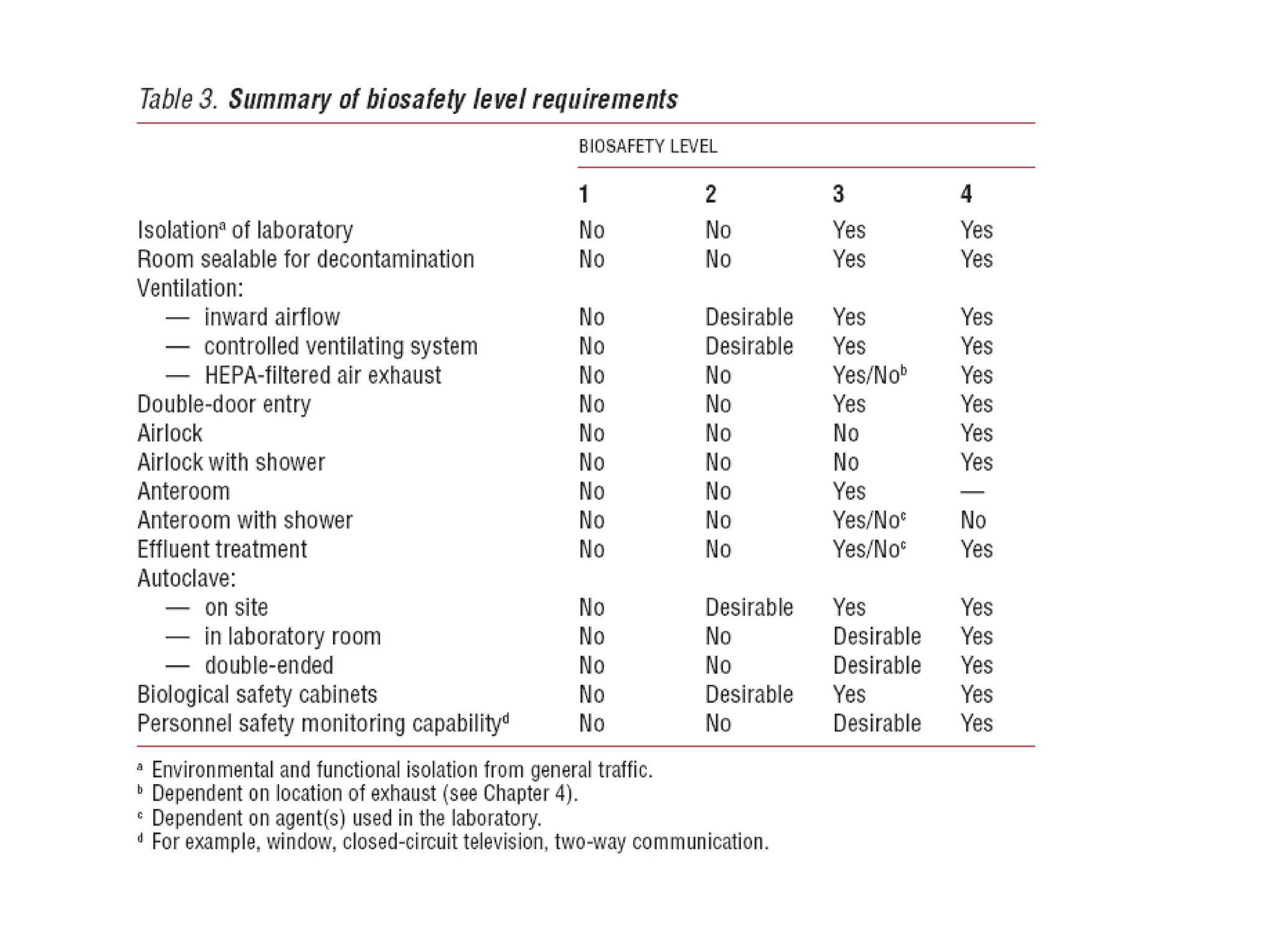
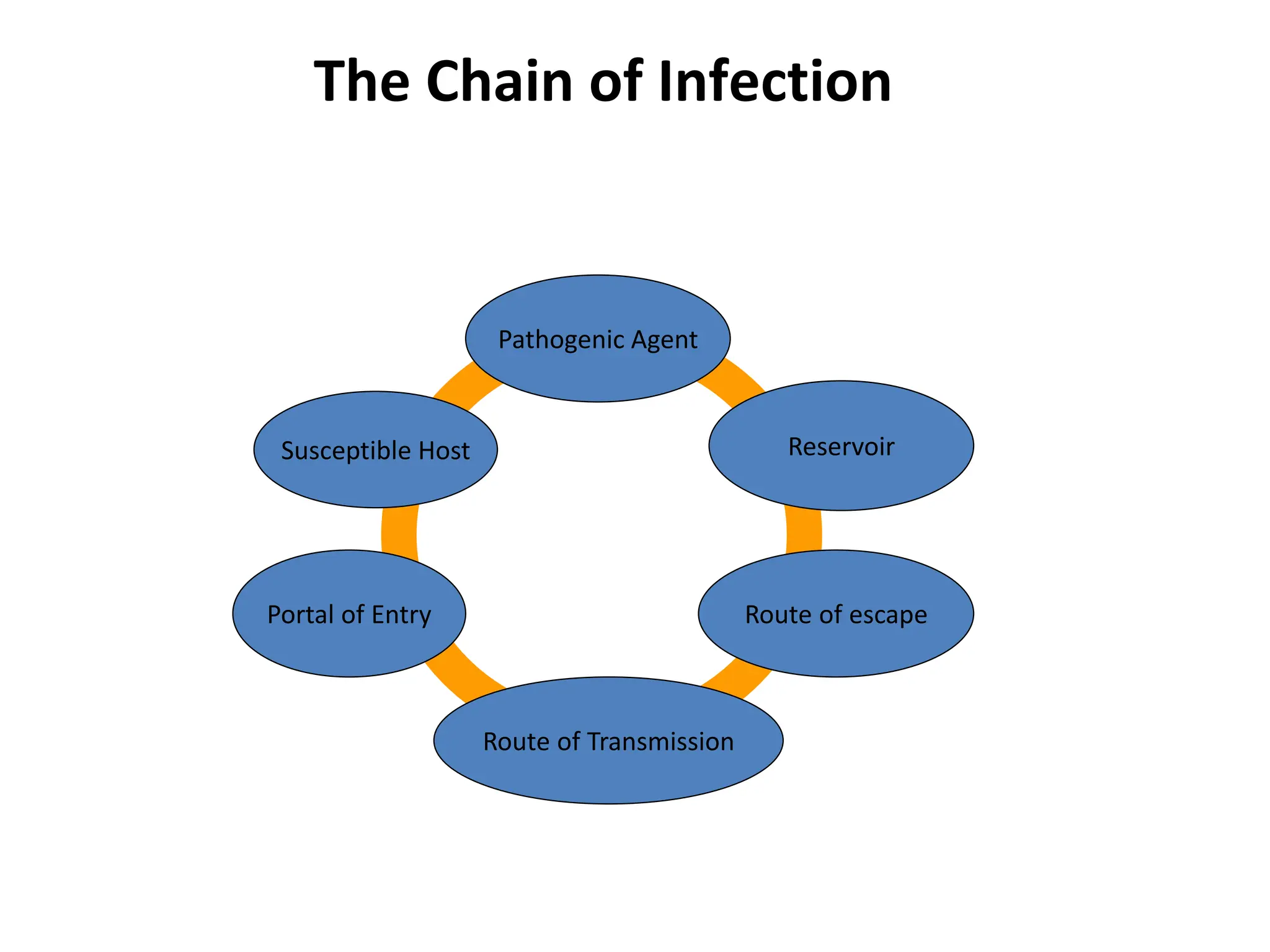
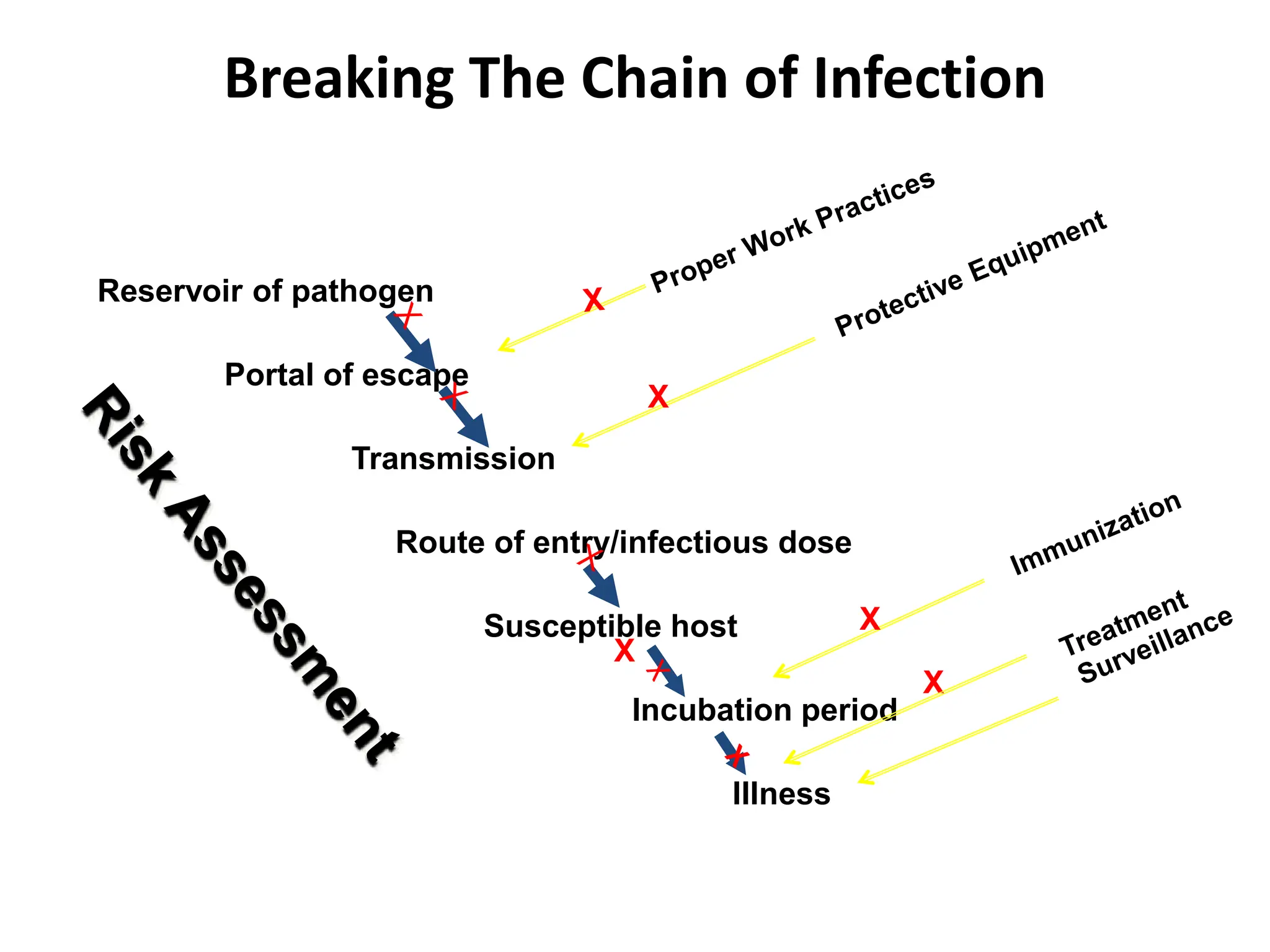
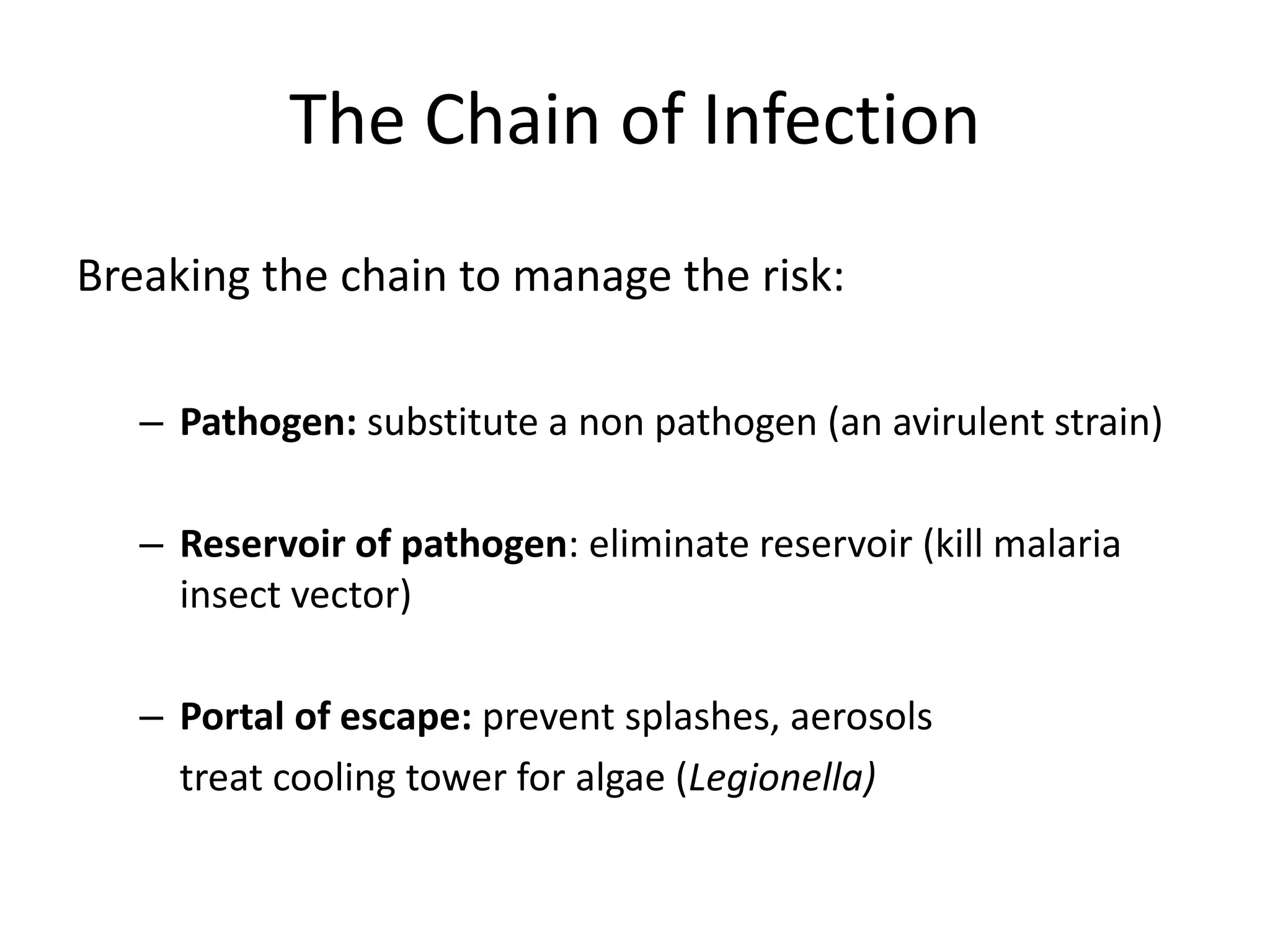
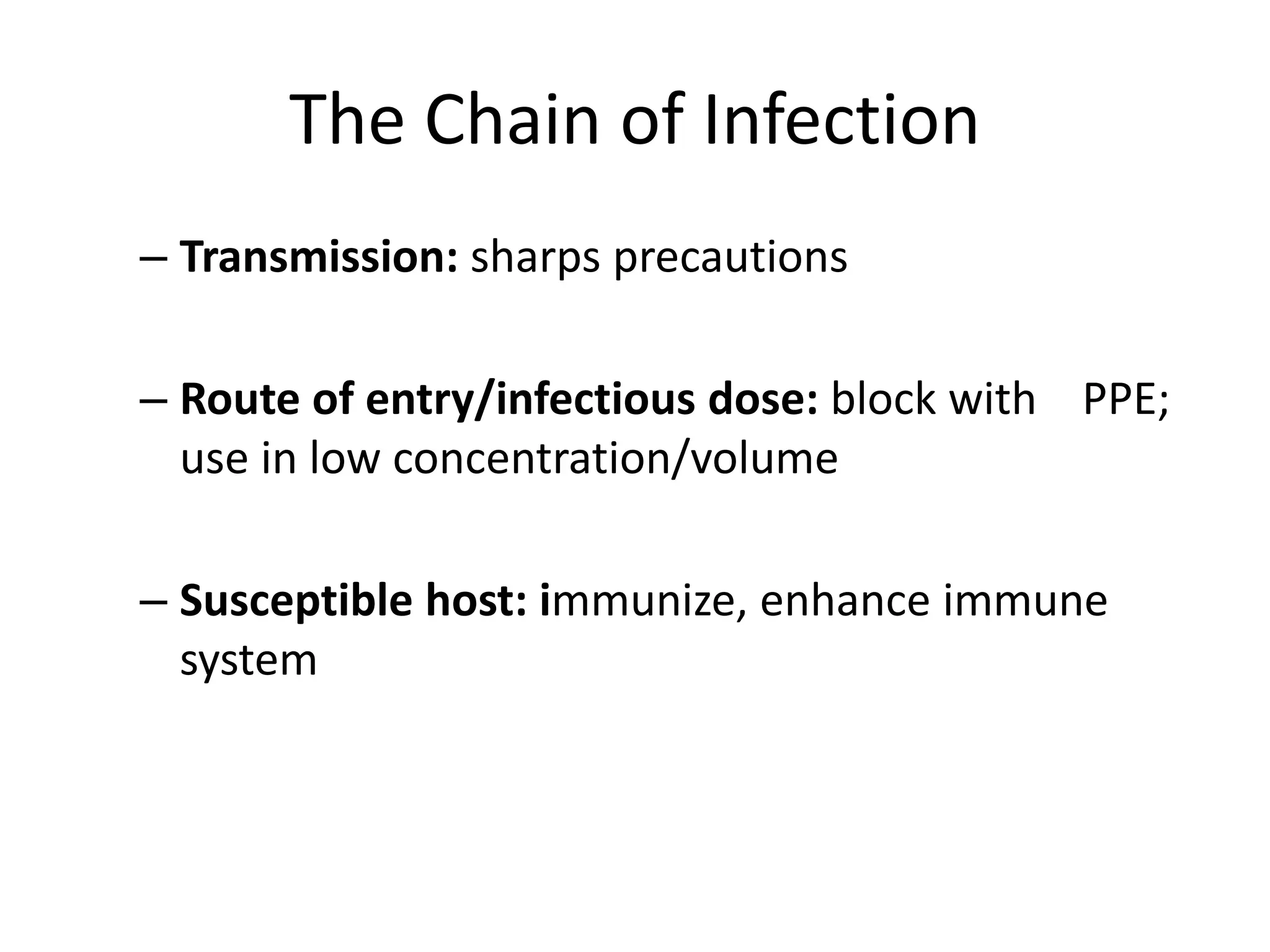
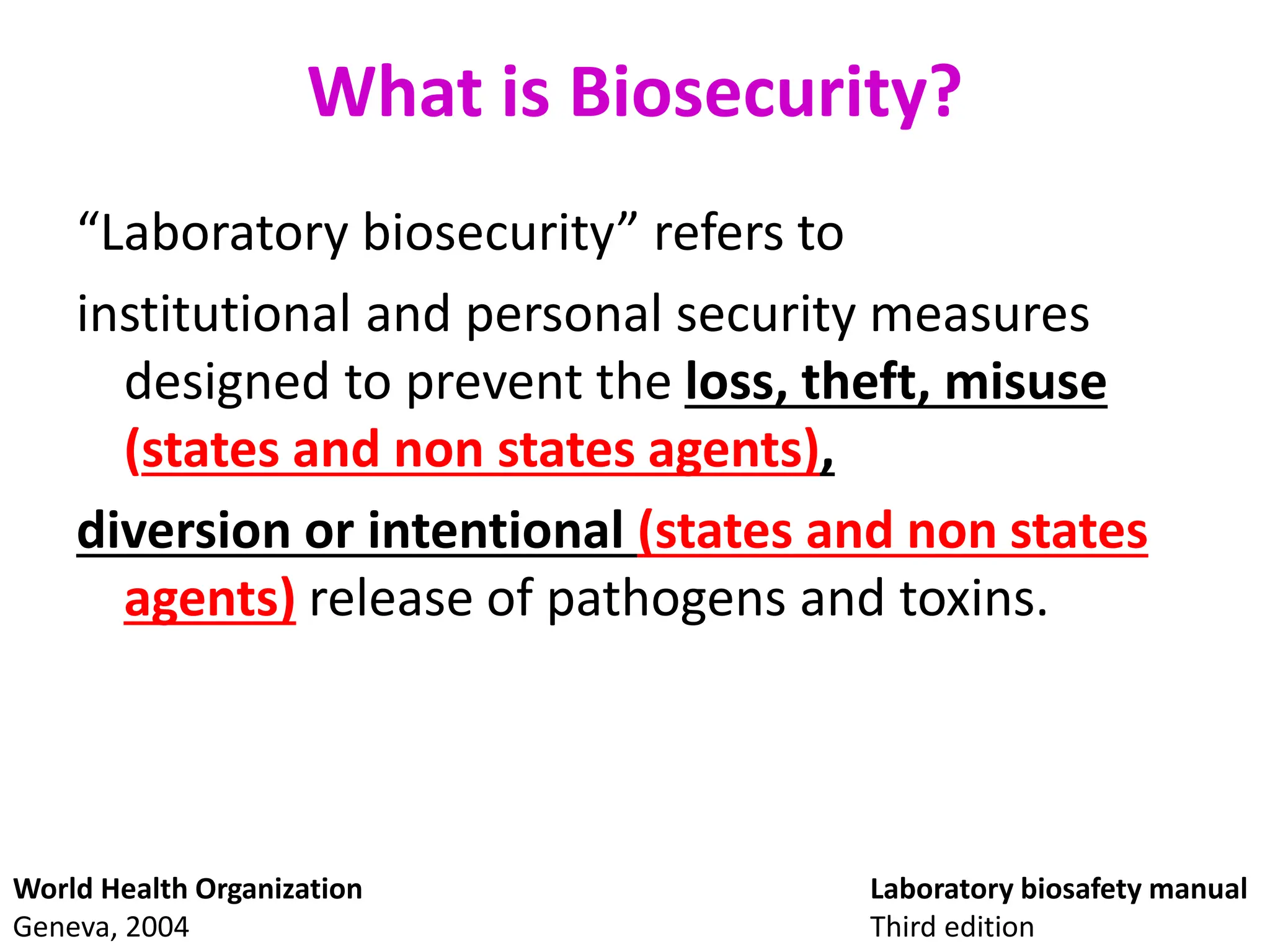
This document discusses biosafety and biosecurity. It defines biosafety as containment principles and practices to prevent unintentional exposure to pathogens. This includes laboratory worker protection, containment design, guidelines and safe practices. It describes World Health Organization (WHO) risk groups 1-4 which categorize agents based on factors like pathogenicity. It also outlines biosafety levels 1-4 which are determined by composite factors including containment and procedures. The document emphasizes principles like risk assessment, training, and emergency response planning. It defines biosecurity as measures to prevent theft or intentional release of pathogens. Developing strong biosafety and biosecurity programs requires involvement from various stakeholders.