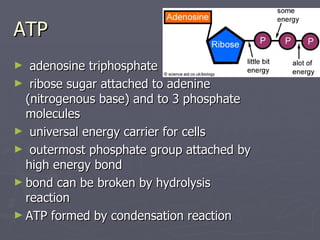
The document discusses 4 types of biochemical reactions: neutralization, redox, hydrolysis, and condensation. Neutralization involves acids and bases reacting to form salts and water. Redox reactions involve the transfer of electrons between reactants, with one being oxidized and one being reduced. Hydrolysis reactions involve adding water to break bonds, while condensation reactions bond molecules together and release water. These reaction types are used in important biological processes like cellular respiration and carbohydrate and lipid synthesis.