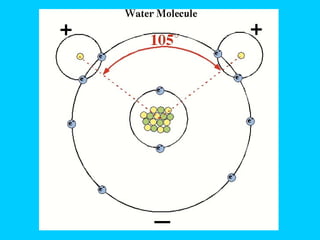
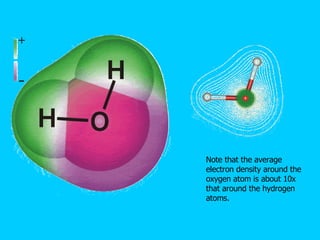
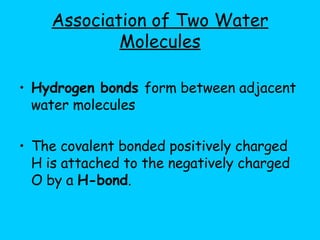
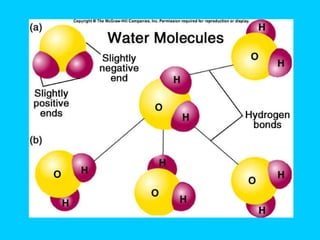
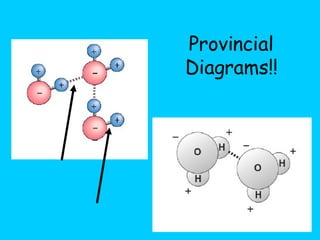
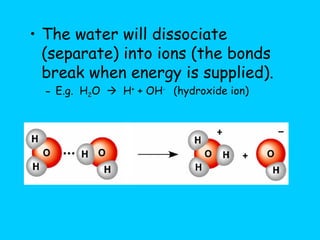
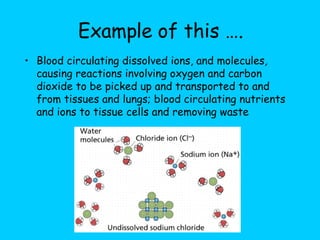
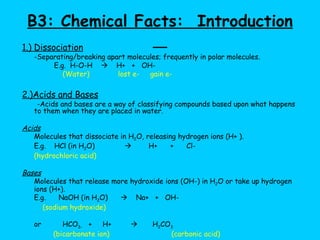
Water makes up a large percentage of living organisms and plays several important roles. It is a polar molecule that forms hydrogen bonds, giving it properties like being an excellent solvent, temperature regulator, and lubricant that are beneficial for life. Biological systems also rely on maintaining precise pH levels, which is influenced by acids, bases, and buffers. Buffers help stabilize pH by neutralizing excess hydrogen or hydroxide ions through various chemical reactions important for biological functions.








![3.) pH : A scale (-log[H+]) that measures the [H+] (hydrogen ion concentration). Indicates the strength of an acid or basic/alkaline solution. [H+] acidic [OH-]basic/alkaline 0 7 14 Less than 7 = an acid Greater than 7 = a base/alkaline 7 = Neutral pH scale runs in increments of 10. E.g. 1x10-4 [H+] = pH 4 ( acidic ) 1x10-7 [H+] = pH 7 ( neutral ), such as water 1x10-9 [H+] = pH 9 ( basic/alkaline ) Animations of pH http://www.purchon.com/chemistry/ph.htm http://www.johnkyrk.com/pH.html](https://image.slidesharecdn.com/b2-3-1203474445269318-4/85/B2-3-19-320.jpg)

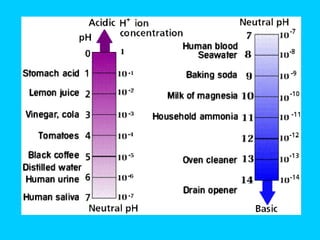
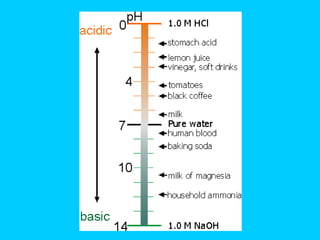
![4.) Strengths The strength of an acid depends on the [H+] ions and the strength of a base depends on the [OH - ] ions. Therefore, the stronger the acid, the greater the [H+]; the stronger the base , the greater the [OH-].](https://image.slidesharecdn.com/b2-3-1203474445269318-4/85/B2-3-23-320.jpg)

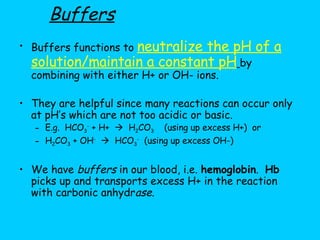
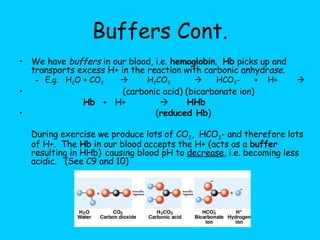
![6.) Buffers Slight changes in pH can be harmful. A buffer will minimize changes in [H+] and/or [OH-] by taking up excess H+ or OH- or donating H+ or OH-. (A substance that acts as a hydrogen ion "sponge" and prevents drastic changes in pH when acid is added.)](https://image.slidesharecdn.com/b2-3-1203474445269318-4/85/B2-3-25-320.jpg)