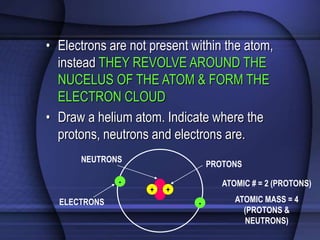
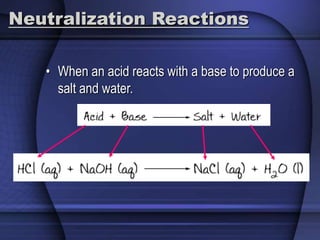
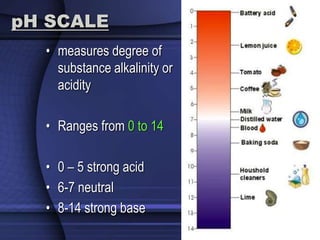
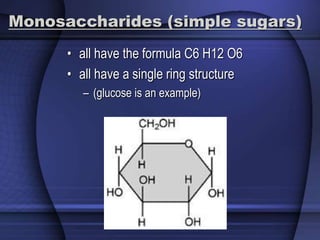
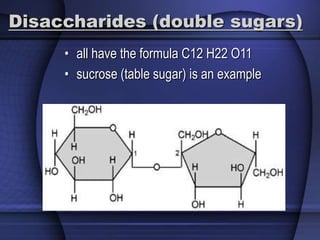
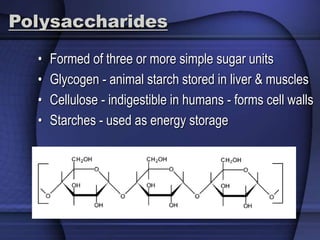
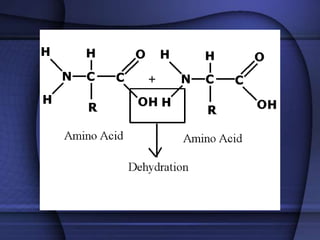
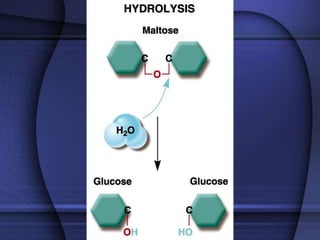
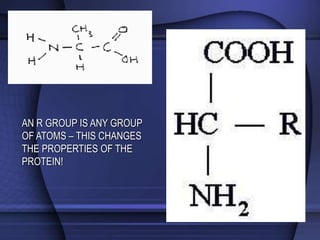
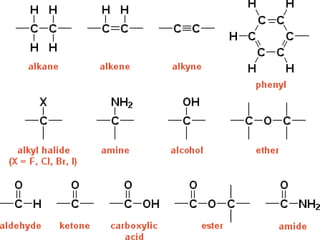
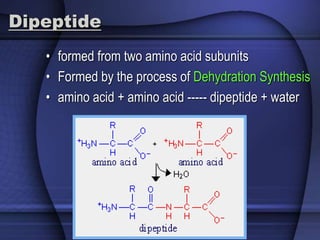
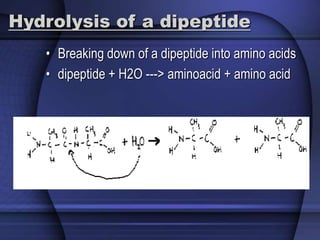
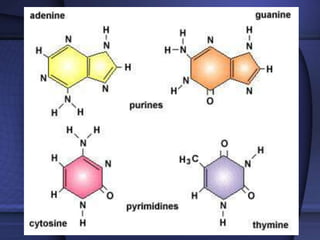
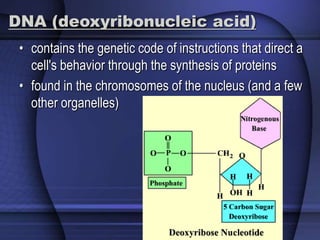
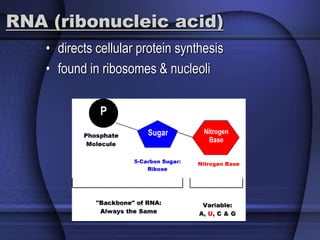
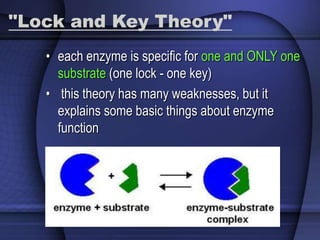
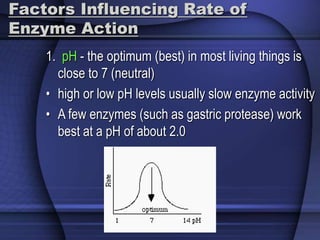
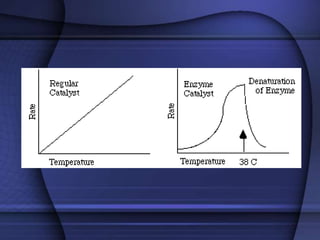
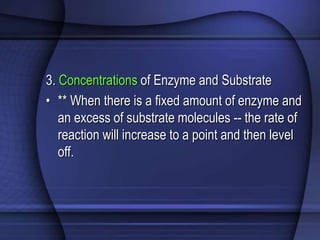
The document provides an overview of biochemistry and chemistry concepts. It discusses the basic units of matter like elements and atoms. It then explains chemical bonds, compounds, and mixtures. Key biomolecules like carbohydrates, lipids, proteins, and nucleic acids are introduced along with their structures and functions. Finally, it briefly covers chemical reactions, enzymes, and pH.