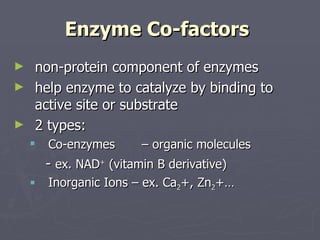
Enzymes are proteins that act as biological catalysts, speeding up chemical reactions in living things. They have an active site that attracts and binds to specific substrate molecules. Once bound, the enzyme-substrate complex undergoes a shape change that either breaks or forms bonds in the substrate. This releases the product and returns the enzyme to its original shape to catalyze more reactions. Enzymes lower the activation energy needed for reactions and are regulated through cofactors, inhibitors, feedback inhibition, and subcellular localization.