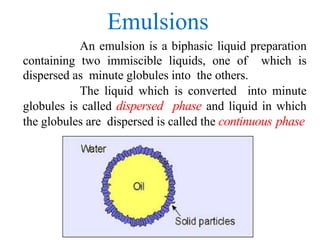
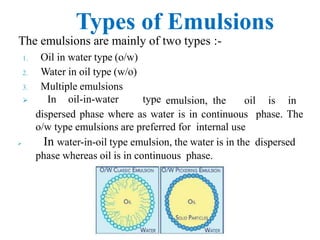
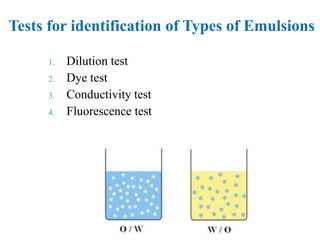
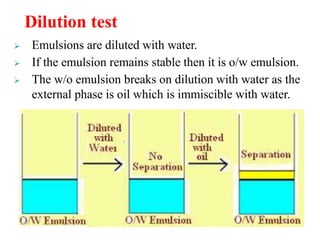
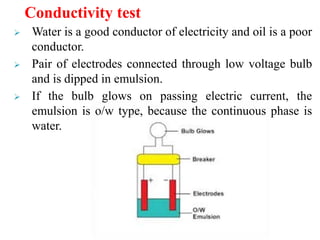
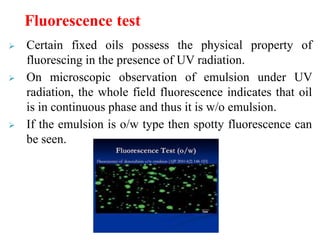
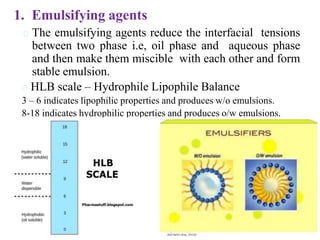
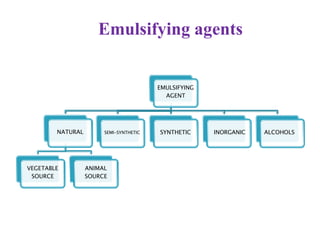
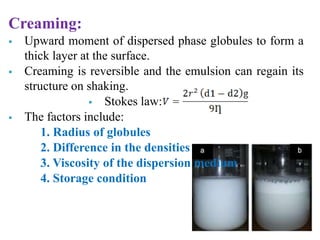
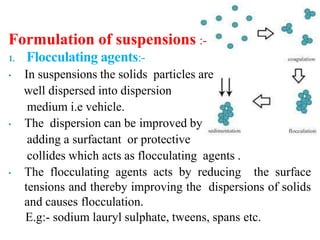
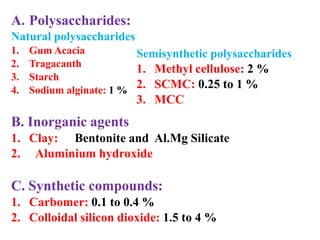
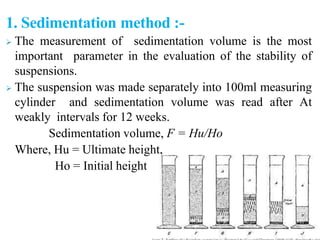
This document discusses emulsions and suspensions. It defines emulsions as biphasic liquid preparations containing two immiscible liquids, one dispersed as globules in the other. Suspensions are biphasic preparations with finely divided solids dispersed in a liquid vehicle. The document describes the types, formulations, evaluation and factors affecting stability of emulsions and suspensions. It also provides details of various tests to identify the types of emulsions.