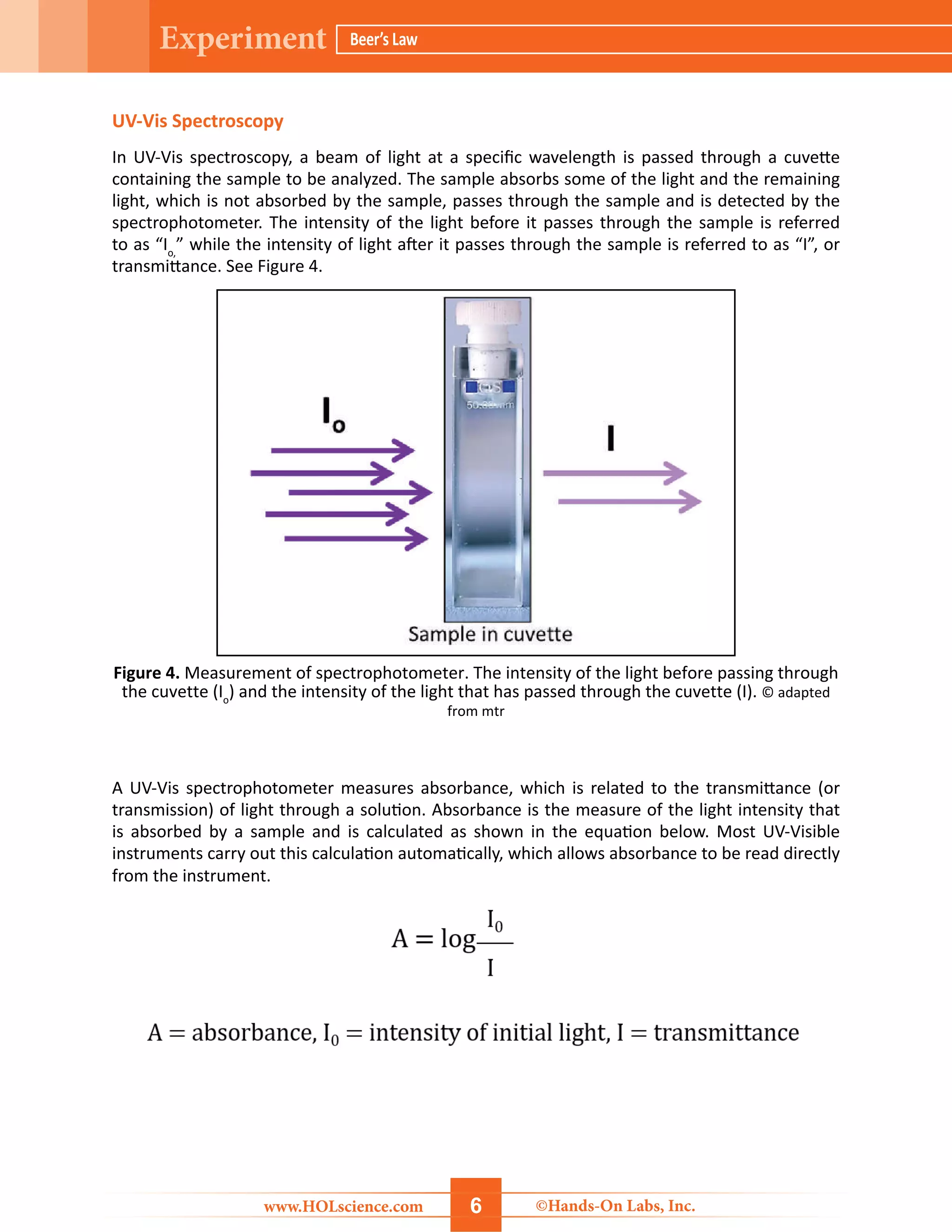
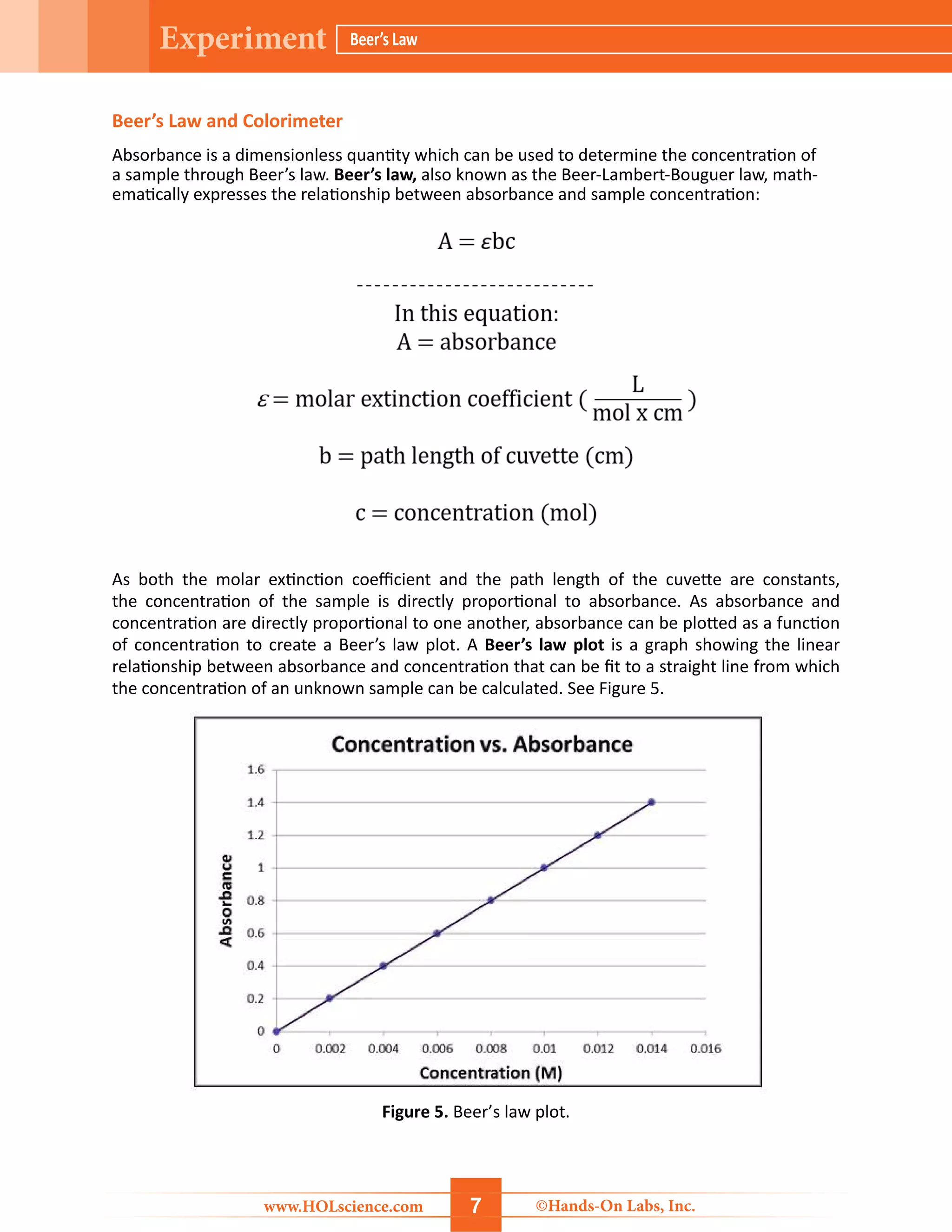
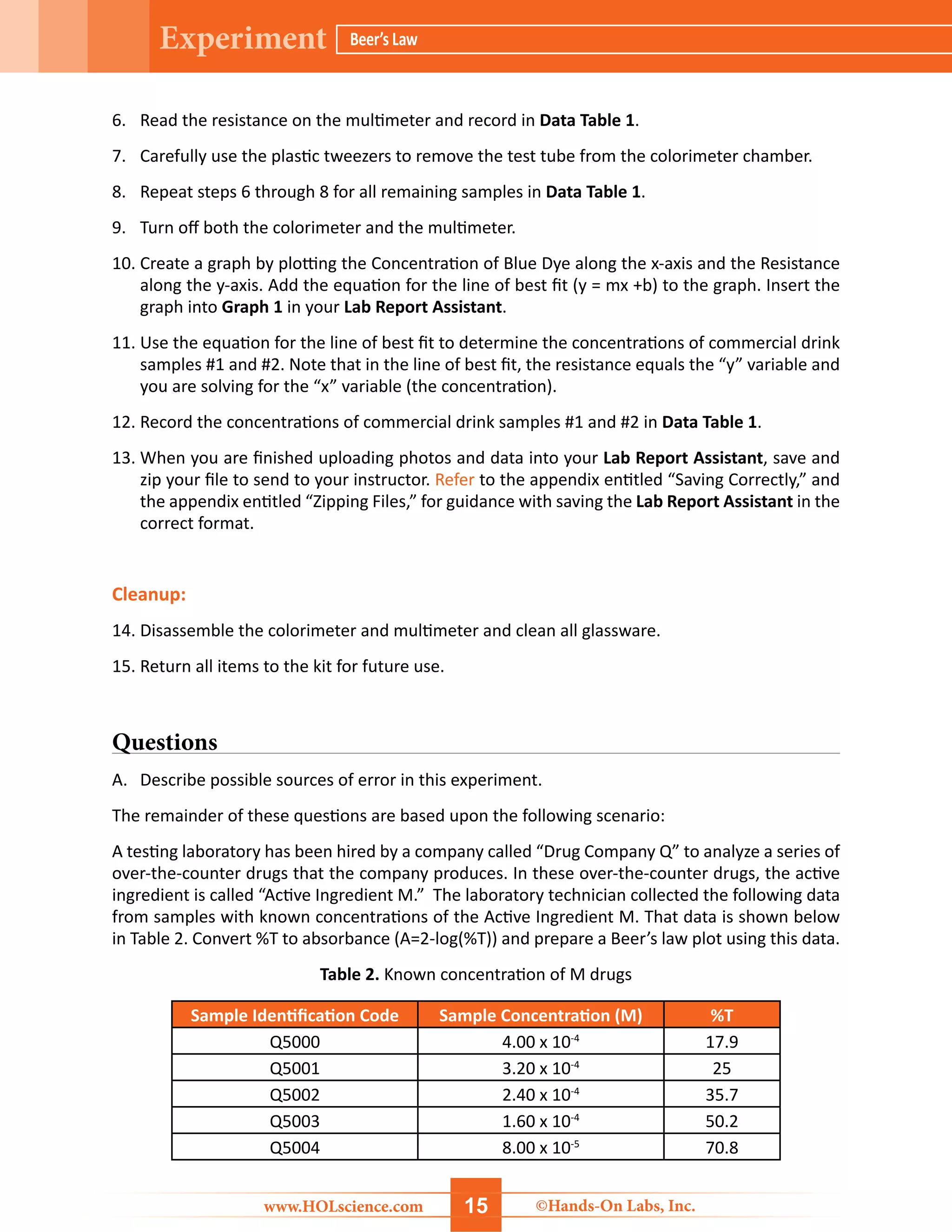
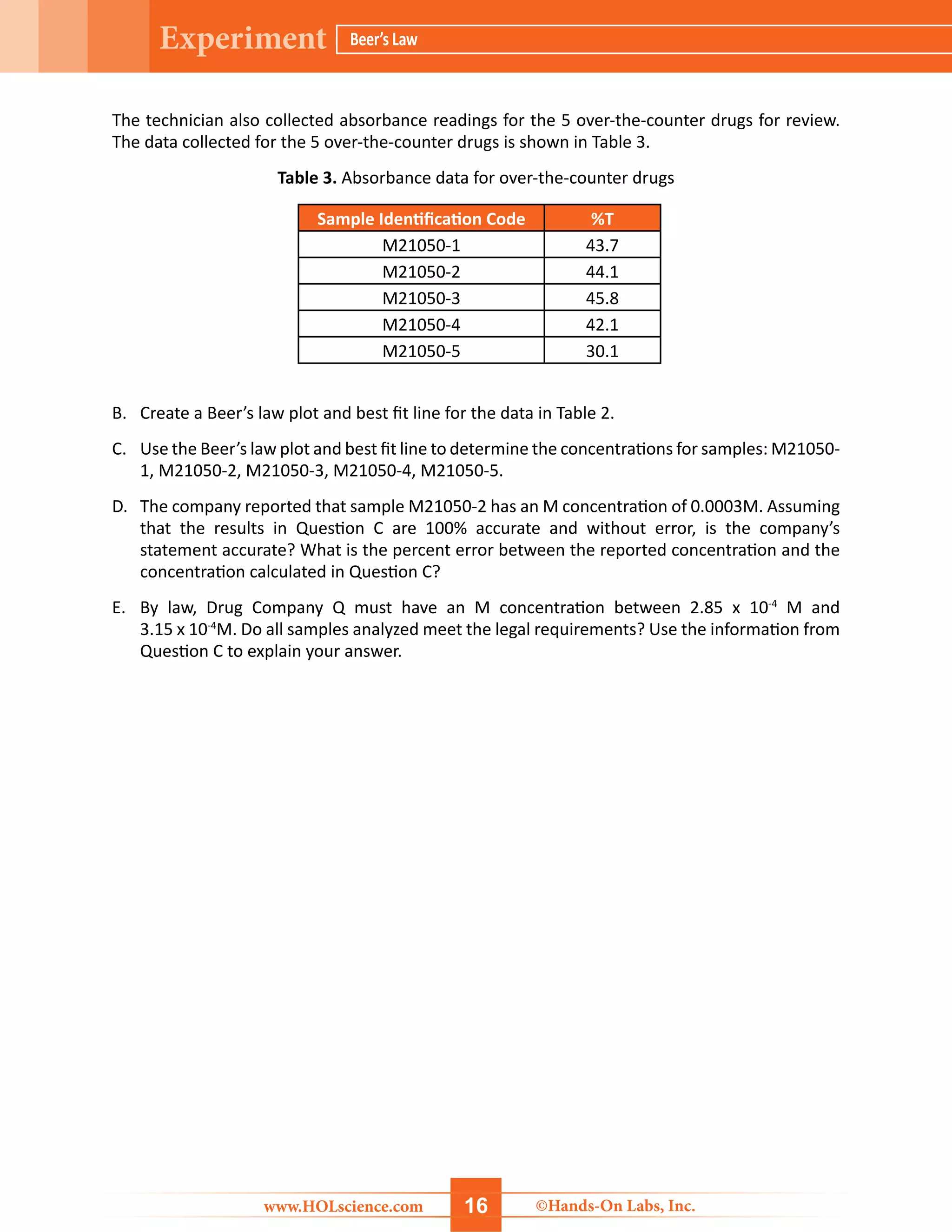
The document details a hands-on laboratory experiment focusing on Beer’s Law, which correlates absorbance and concentration in solutions. Students will learn about spectroscopy, prepare samples, and utilize a colorimeter to create a Beer’s Law plot to determine the concentration of unknown samples. Safety precautions and a thorough materials list are provided, along with step-by-step procedures for conducting the experiment.