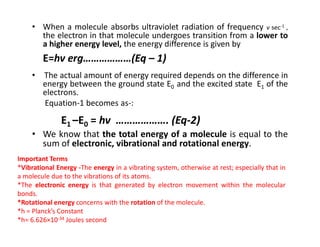
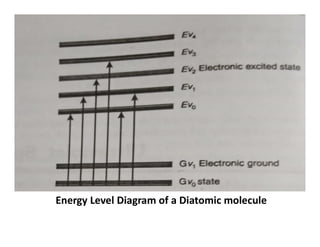
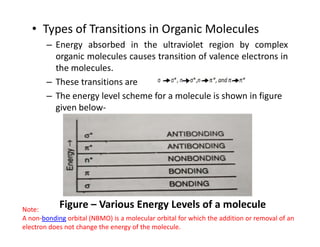
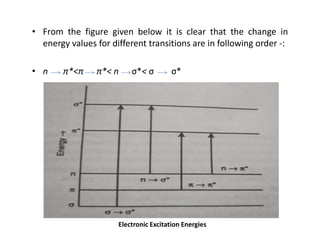
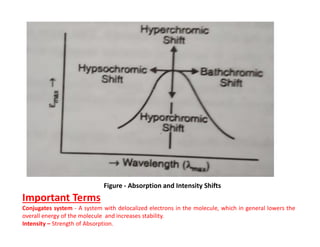
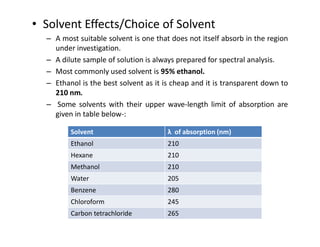
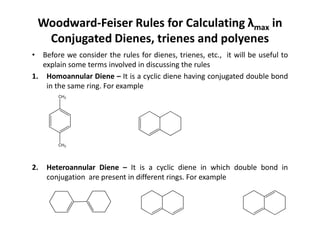
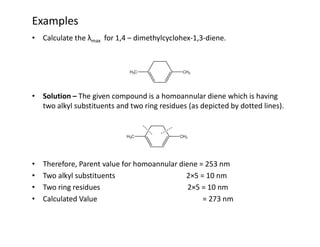
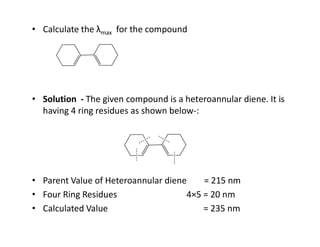
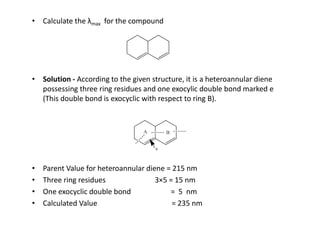
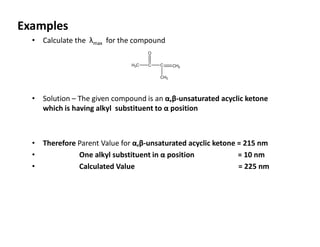
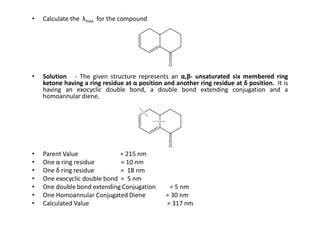
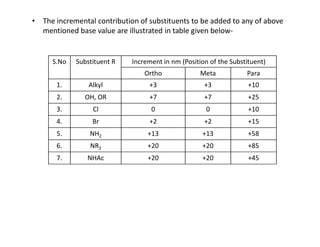
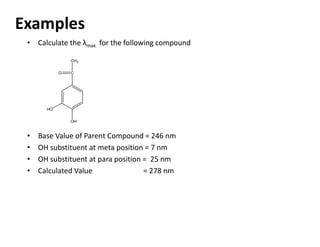
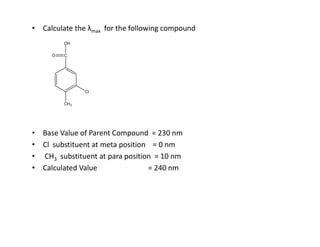
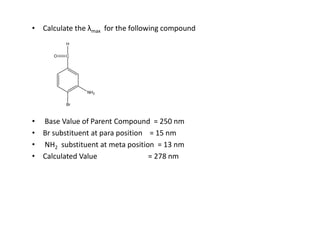
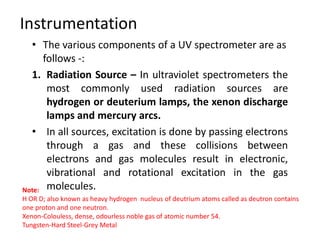
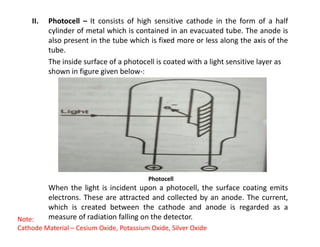
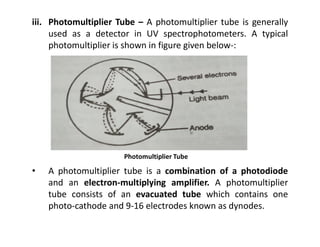
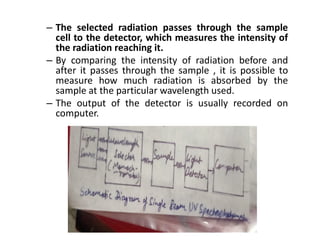
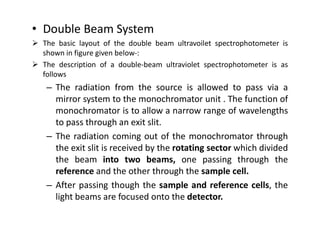
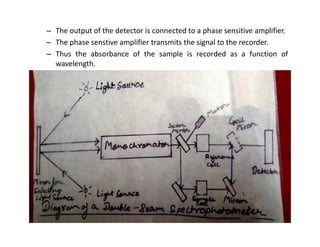
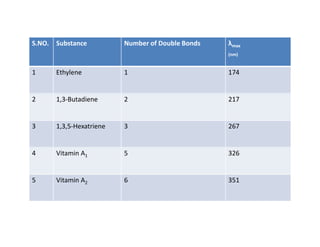
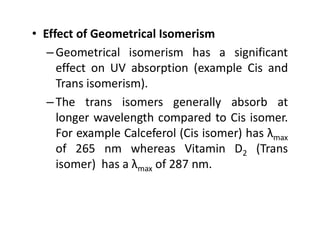
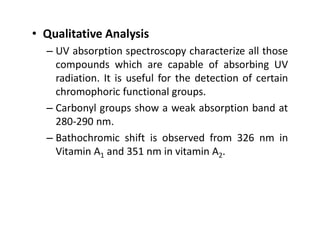
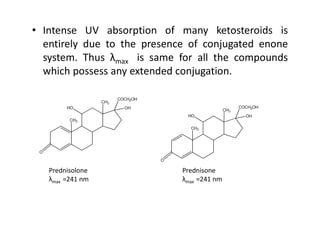
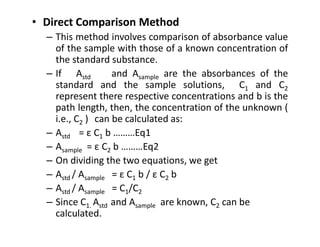
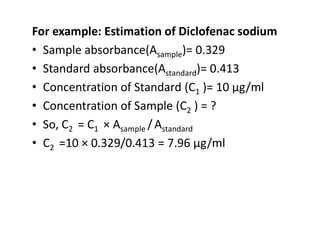
Ultraviolet spectroscopy involves the absorption of UV radiation by molecules, causing electronic transitions in valence electrons. The UV region is divided into near (2000-4000 Å) and far/vacuum (below 2000 Å) regions. UV absorption spectra arise from transitions of electrons between lower and higher electronic energy levels. Important terms include chromophores, which absorb UV radiation, and auxochromes, which shift absorption maxima. Woodward-Feiser rules can be used to calculate absorption maxima based on molecular structure. Common instrumentation includes hydrogen and deuterium lamps as well as mercury arcs as UV sources.