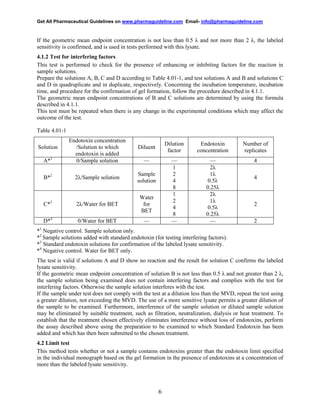
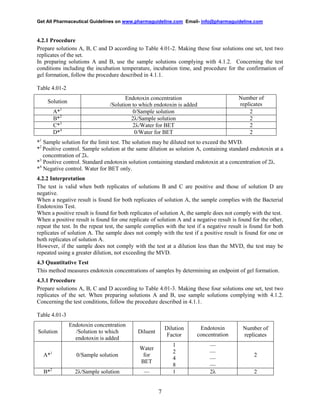
This document provides a draft consensus guideline from the International Conference on Harmonisation (ICH) regarding the evaluation and recommendation of pharmacopoeial texts for the bacterial endotoxins test general chapter. It recommends that three analytical procedures from the European, Japanese, and United States pharmacopoeias can be used interchangeably. Acceptance criteria are not specified in the evaluated texts and should be included in product dossiers. The annex provides implementation considerations for regulatory authorities in ICH regions.