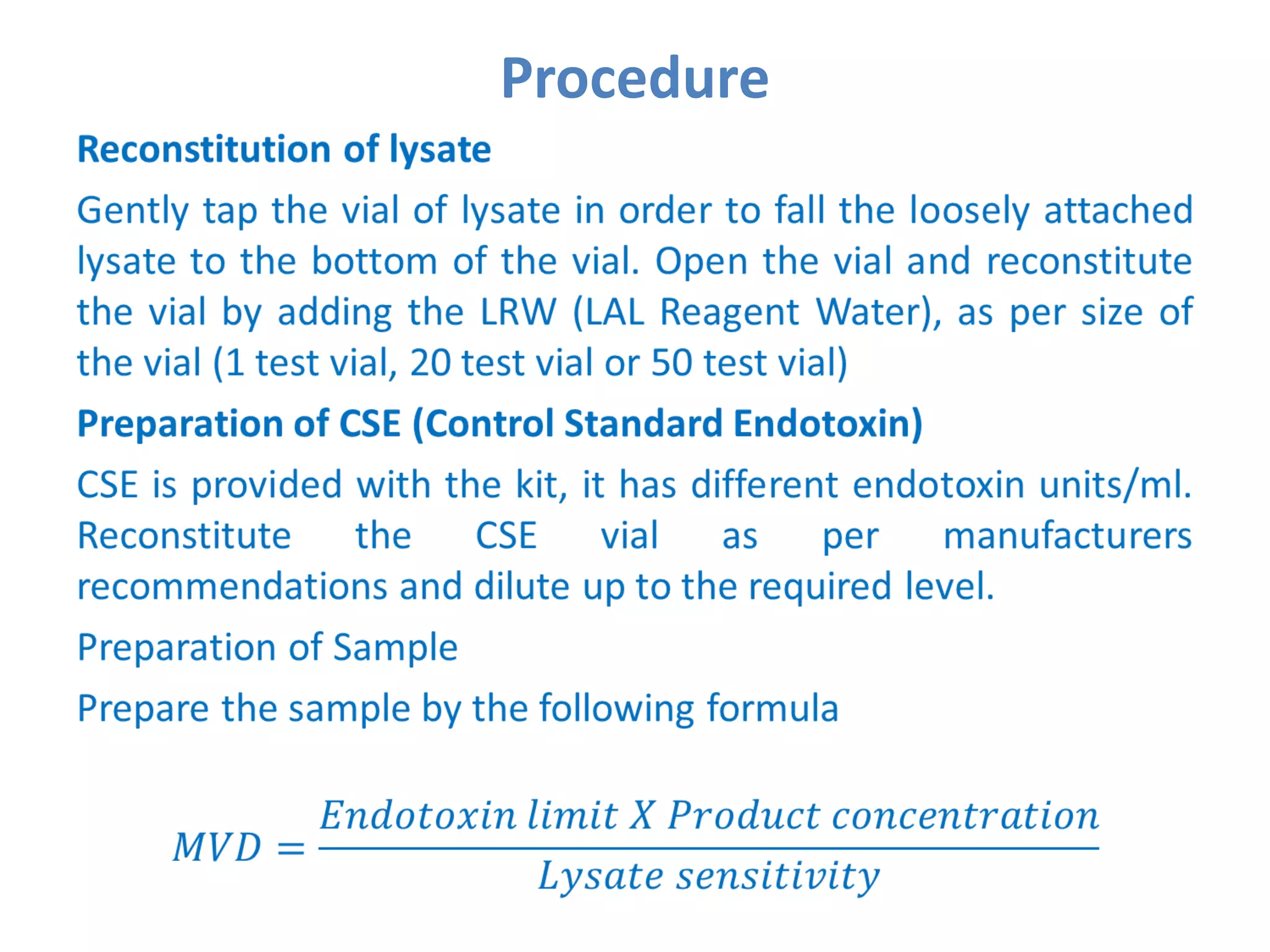
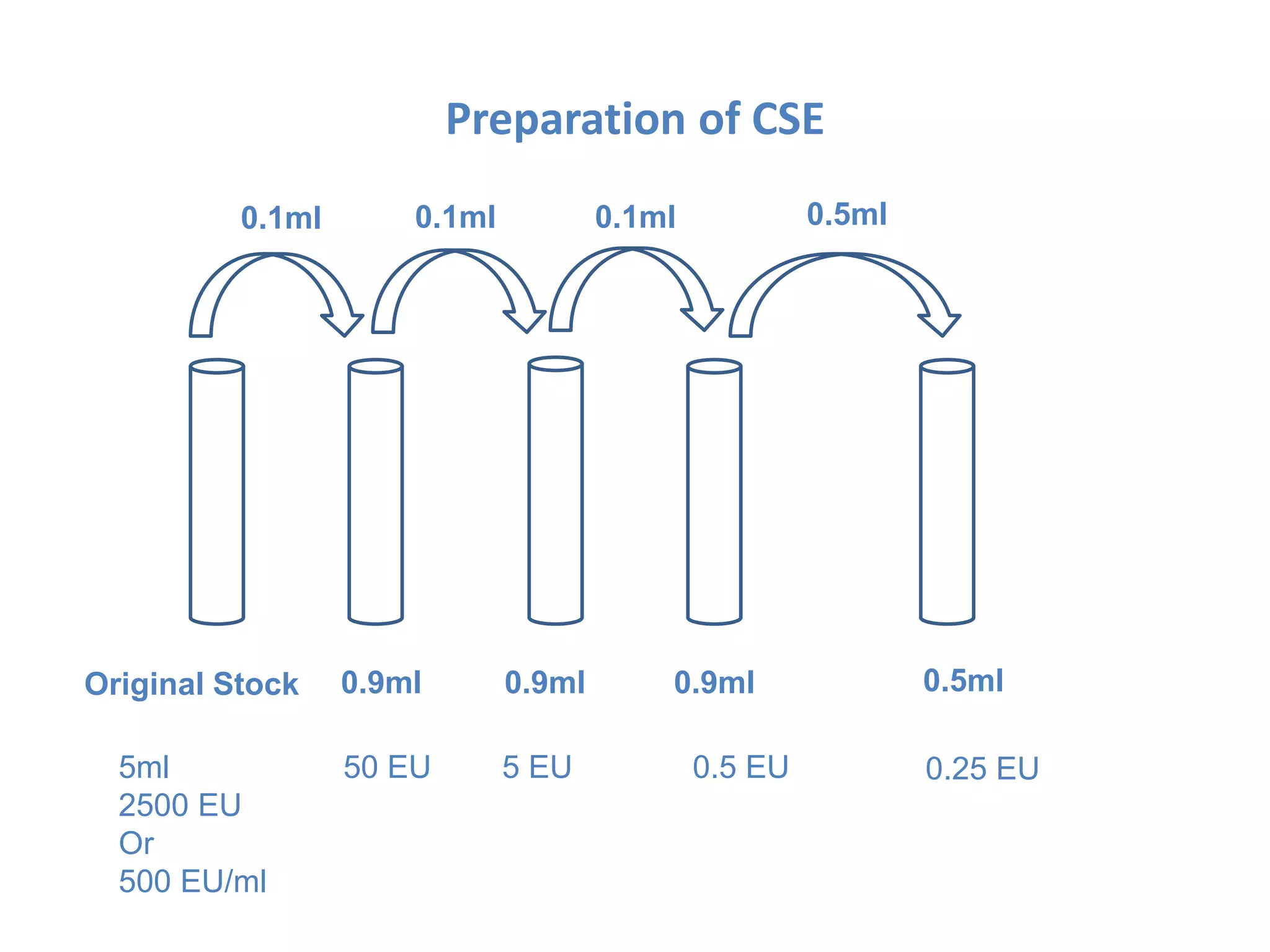
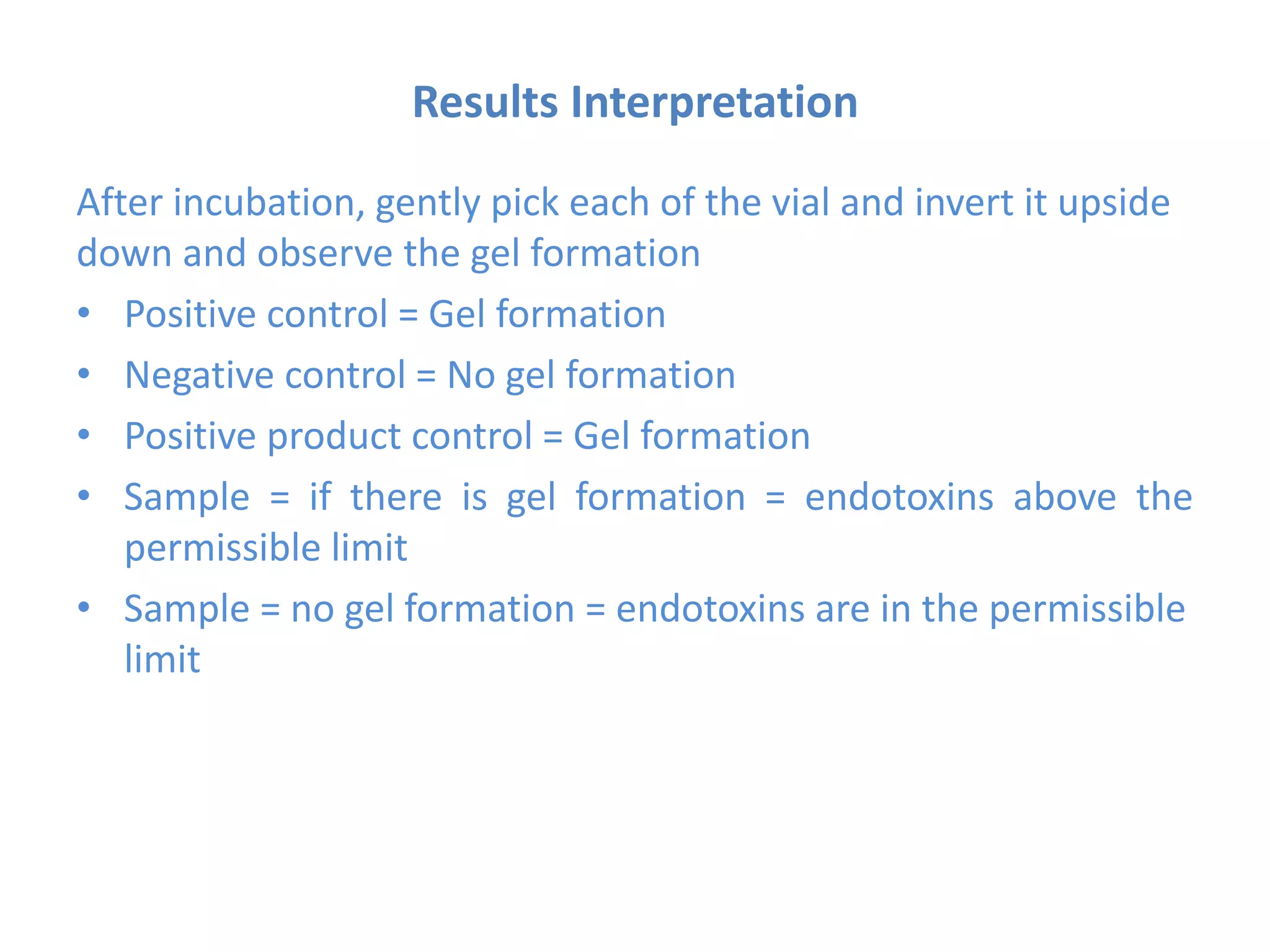
This document describes the Limulus Amoebocyte Lysate (LAL) test used to detect bacterial endotoxins. It involves mixing the LAL reagent, obtained from horseshoe crab blood, with test samples. If endotoxins are present, the LAL reagent will clot, forming a gel. The procedure involves incubating mixtures of the LAL reagent with test samples, positive controls containing endotoxins, and negative controls. After incubation, the mixtures are observed to see if gels formed, indicating the presence of endotoxins above permissible limits. The LAL test is a common method used in the pharmaceutical industry to test for bacterial endotoxins in products.