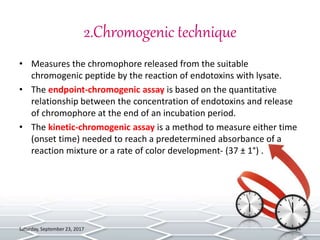
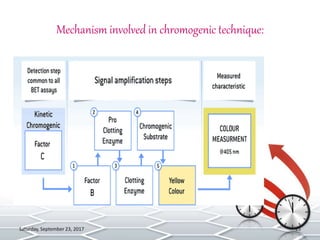
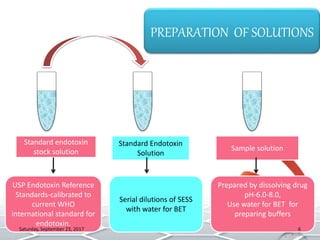
Bacterial endotoxins tests are used to detect and quantify endotoxins from gram-negative bacteria. Endotoxins are heat-stable lipopolysaccharides in bacterial cell walls. The test uses amoebocyte lysate from horseshoe crabs to detect endotoxins. There are several methods to conduct the test including gel clot formation, turbidity measurement, and chromogenic assays. Proper controls and standard curves must be established to validate the test results. The test is used to ensure medical products and devices are free of endotoxins, which can cause adverse reactions in humans.




![• APPARATUS:
• Depyrogenated
• Free of detectable endototxins
• Should not interfere with test
• REAGENTS AND TESTS SOLUTIONS:
• Amoebocyte lysate : Lyophilised
product-lysate of amoebocytes-horseshoe
crab (limulus polyphemus or tachypleus
tridentatus).
• Water for Bacterial Endotoxins
Test: Use water for injection
• Lysate TS: Dissolve Amoebocyte lysate in
water for BET or in buffer recommended
by lysate manufacturer.
• [ Note:use lysate TS having
sensitivity of NLT-0.15
Endotoxin Unit per mL]
Temperature-
250 degrees
Time-30 min’s
Saturday, September 23, 2017 5](https://image.slidesharecdn.com/mahalaxmi-seminar-170923195420/85/Bacterial-Endotoxins-test-5-320.jpg)
![Determination of maximum valid dilution [mvd]
• It is max. allowable dilution at which endotoxin limit can be determined
• Endotoxin limit-for parentals =K/M where K is threshold pyrogenic dose of
endotoxin per Kg of body weight & M is maximum total dose of product per
Kg of body weight
EU/mL ,EU/mg ,EU/unit of biological activity
• Product concentration –mg/mL [in weight-EU/mg]
Endotoxin limit *product concentration
Lysate sensitivity
MVD=
Saturday, September 23, 2017 7](https://image.slidesharecdn.com/mahalaxmi-seminar-170923195420/85/Bacterial-Endotoxins-test-7-320.jpg)
![• V-Maximum dose in mL
• For drugs administrated per square meter of body surface
(Anticancer drugs)-K is EU/m2
Drugs
Radiopharmaceutical
s-formula
Route of administration
K-
(EU/mg)
5
0.2
175(EU/V)
14(EU/V)
Any –Except intrathecal
route
Intrathecal
Determination of maximum valid dilution [mvd]
Saturday, September 23, 2017 8](https://image.slidesharecdn.com/mahalaxmi-seminar-170923195420/85/Bacterial-Endotoxins-test-8-320.jpg)



![1.Preparatory testing
1.Confirmation of labelled lysate sensitivity:
Confirm in 4 replicates –labelled sensitivity( ) - [EU/mL ].
2 1 0.5 0.25 Prepare standard solutions of
at least 4 concentrations
equivalent to 2λ, λ, 0.5λ and
0.25λ by diluting the standard
endotoxin stock solution with
water for BET+0.1mL lysate TS
Transfer to vials-
incubate 37 ± 1°
for 60 ± 2 min)
To test integrity of
gel-invert 180
degrees in one
smooth motion
Positive
Negative
Saturday, September 23, 2017 12](https://image.slidesharecdn.com/mahalaxmi-seminar-170923195420/85/Bacterial-Endotoxins-test-12-320.jpg)