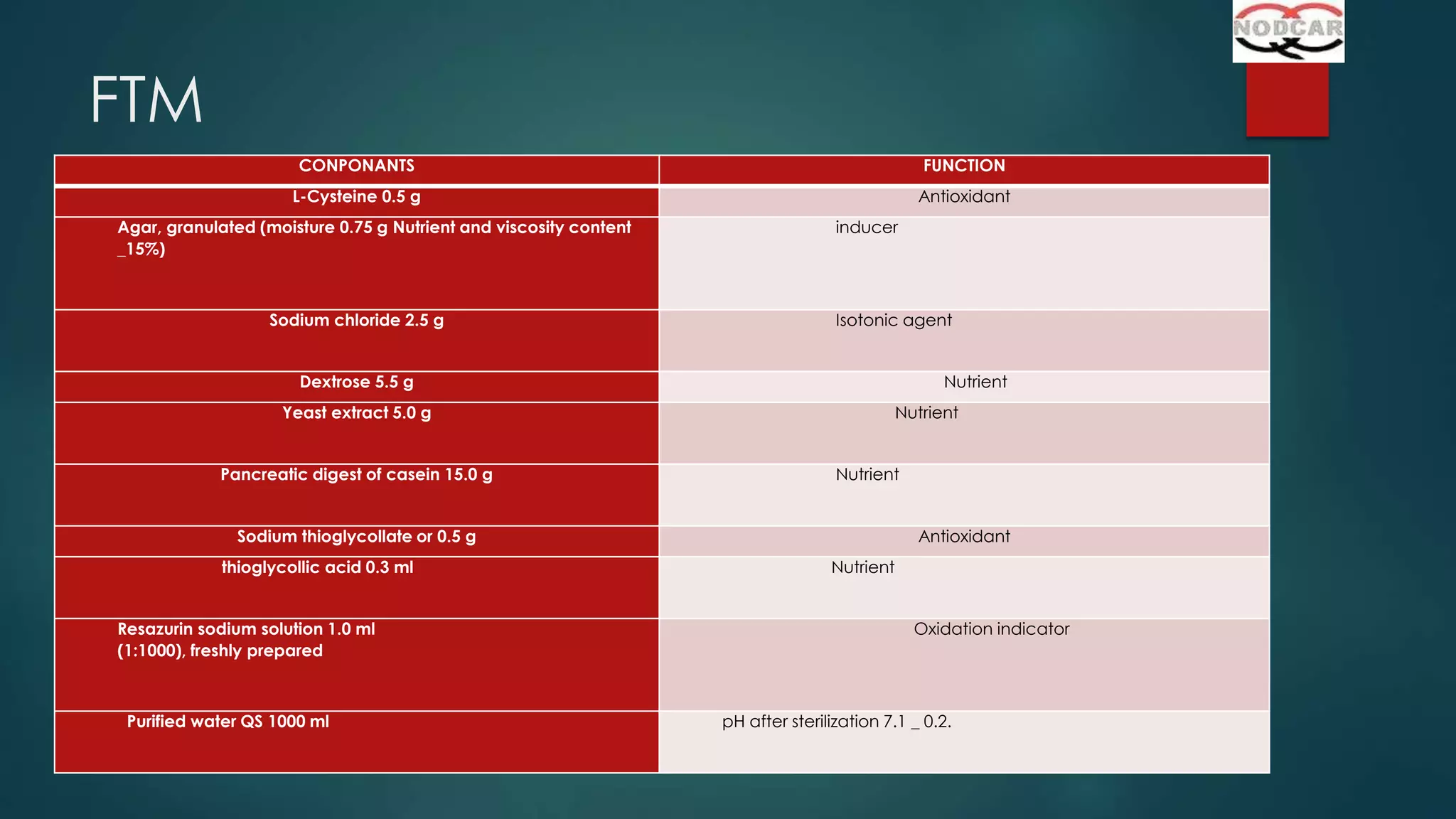
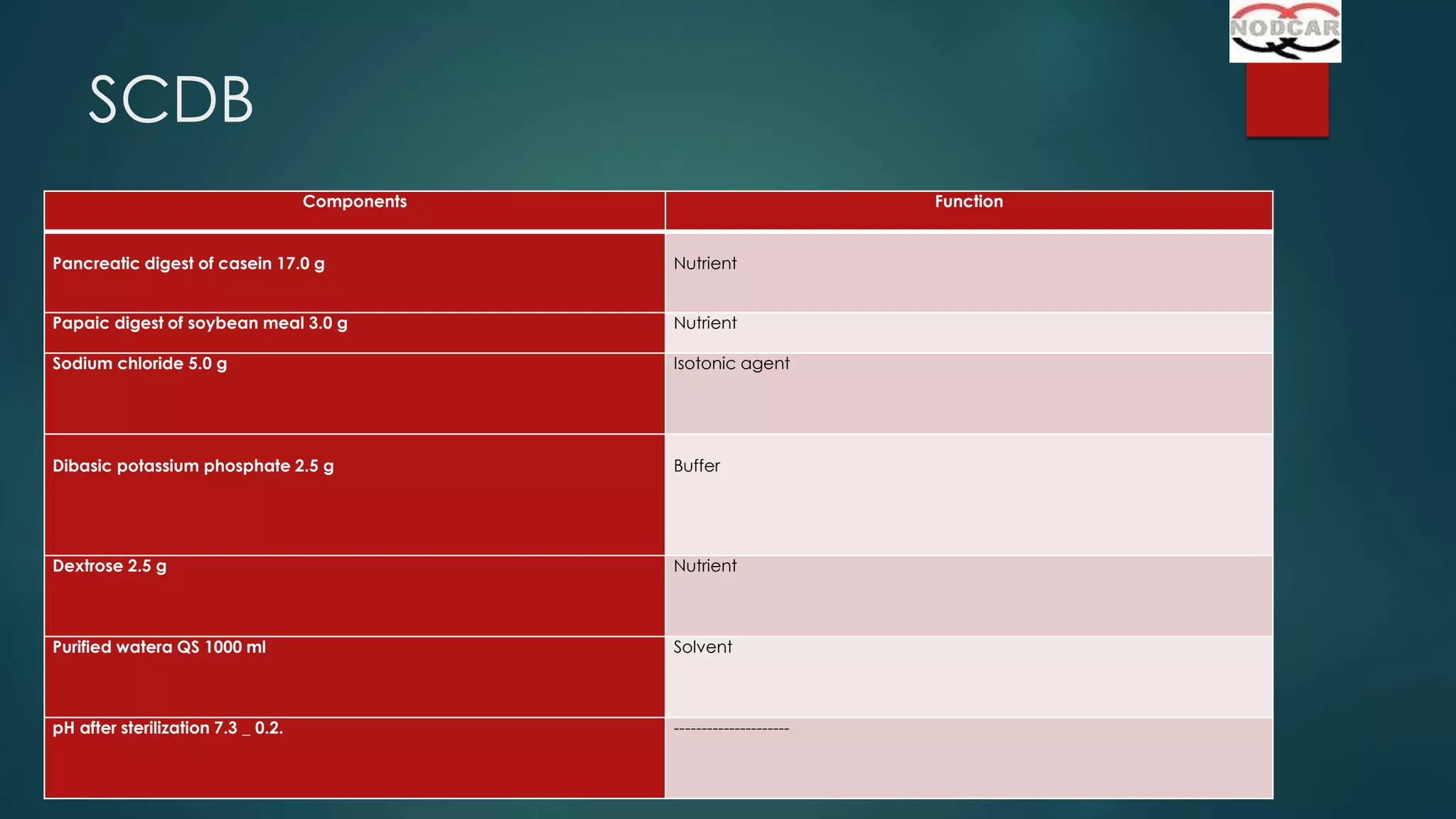
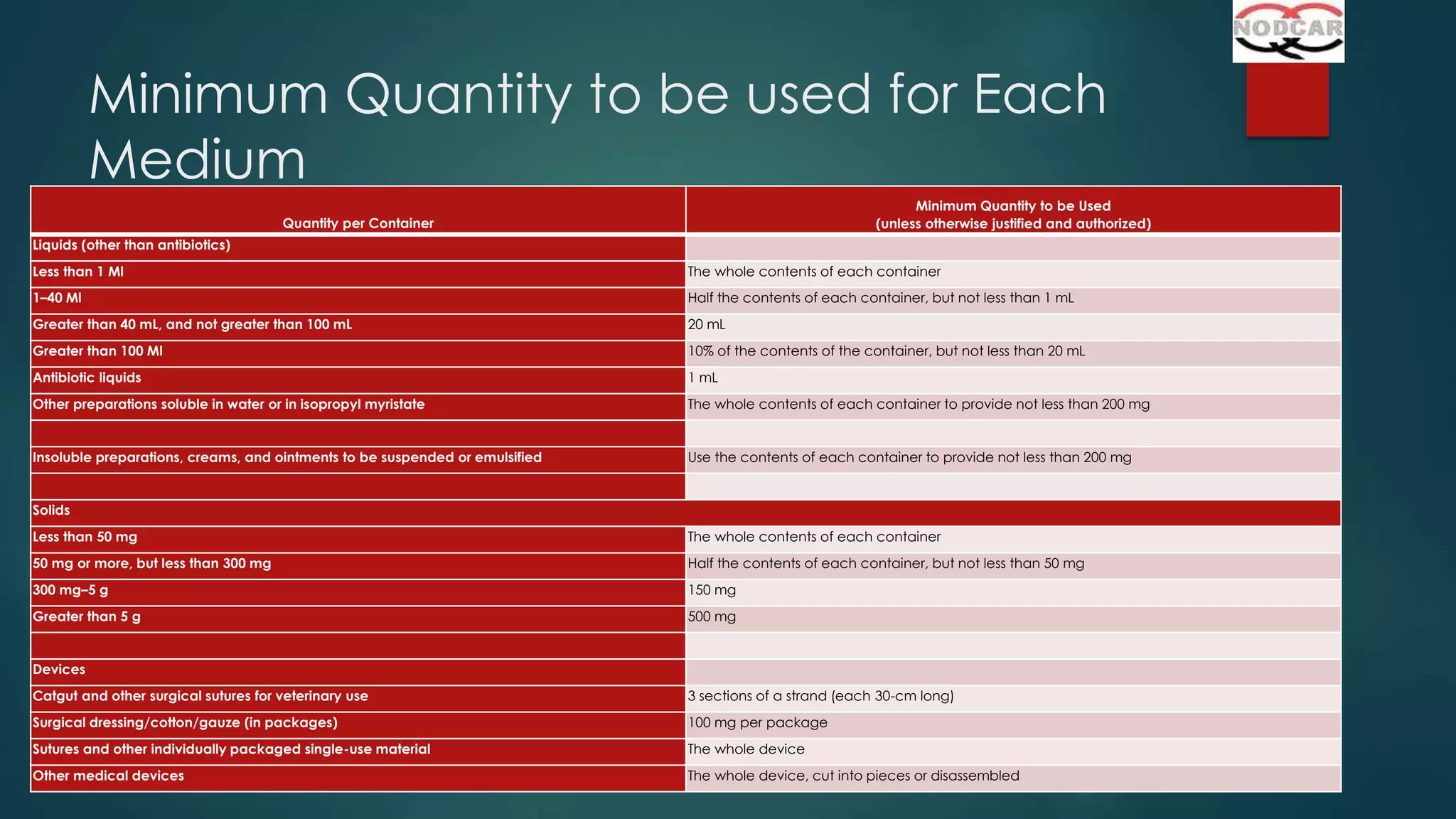
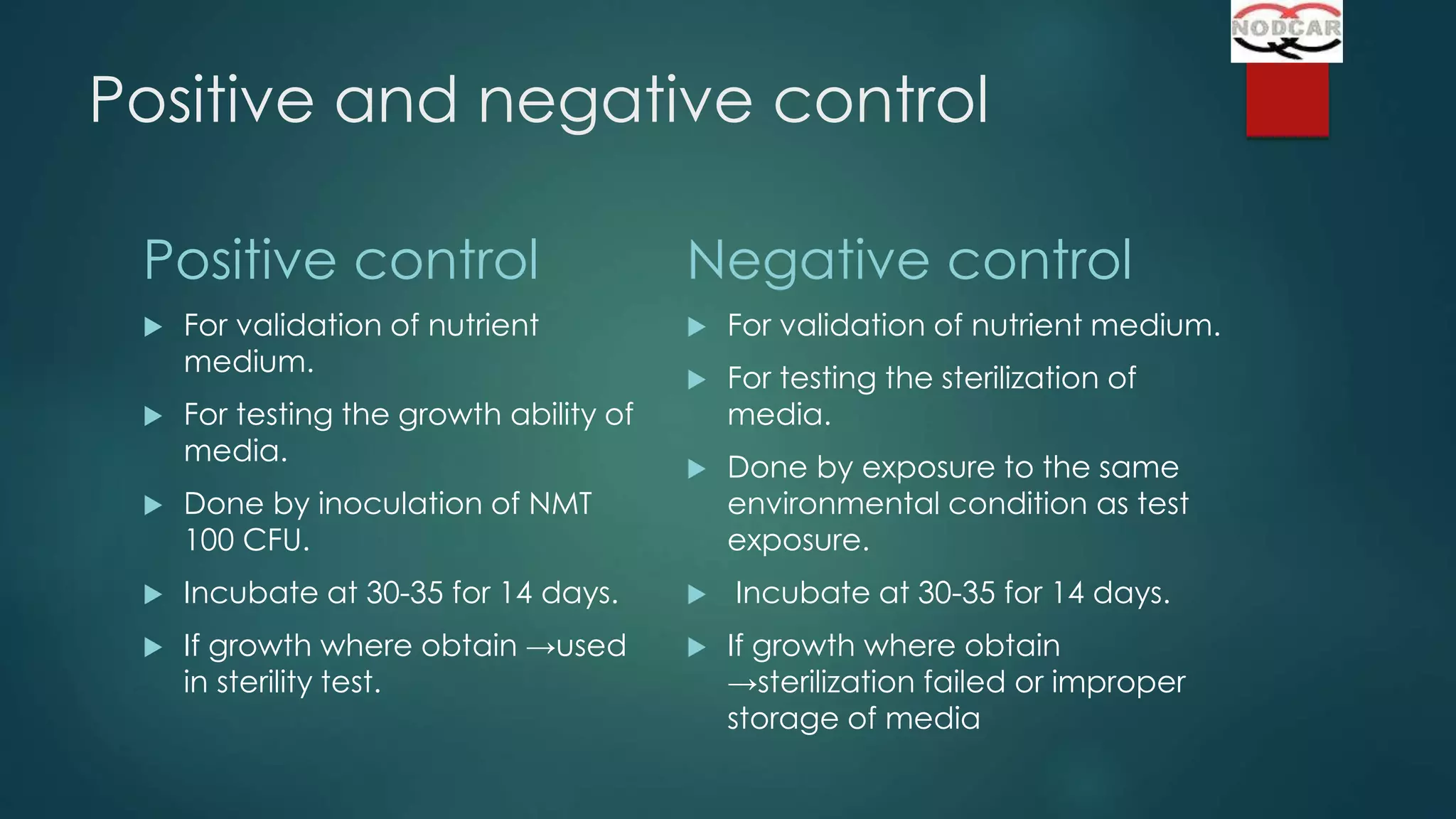
This document provides an overview of sterility testing and rapid microbiological methods. It discusses sterility testing, including definitions, common media used, methods for preparing different types of test products, incubation periods, growth promotion tests, and interpreting results. It also briefly introduces some rapid microbiological methods like ATP bioluminescence, colorimetric growth detection, and cytometry systems. The key purpose of sterility testing is to detect any viable microorganisms in pharmaceutical products or medical devices labeled as sterile.