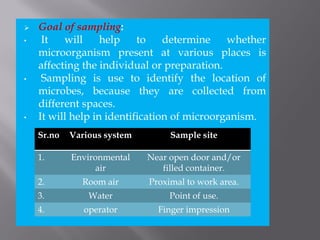
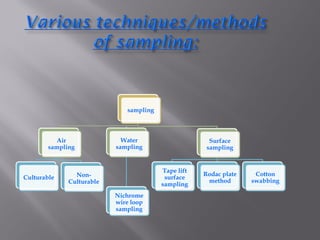
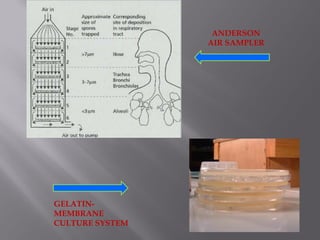
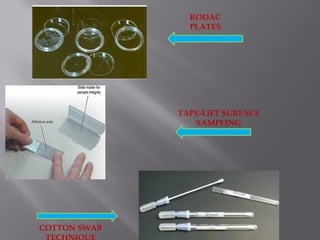
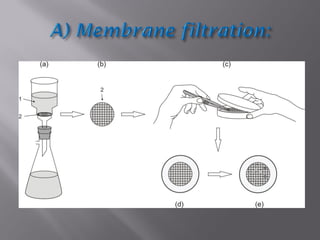
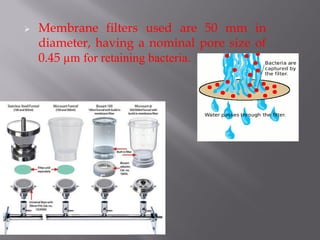
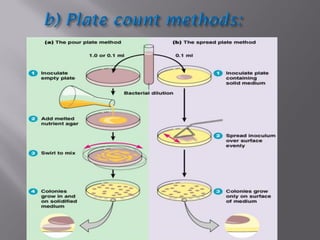
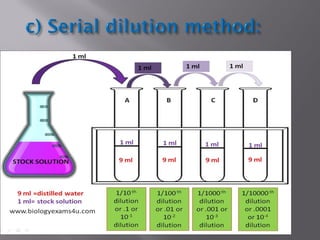
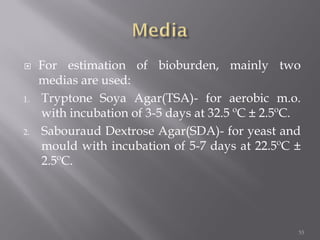
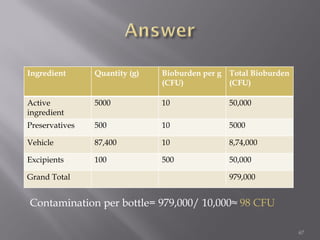
This document discusses bioburden testing, which quantifies the number of microorganisms present on a medical device or pharmaceutical product. It outlines the purposes of bioburden testing such as acting as a quality control measure and determining necessary sterilization doses. The key steps of bioburden testing include sampling techniques, extraction methods, enumeration procedures like plate counting, and incubation. Regulations like CFR 21 and ISO 11737 provide standards for bioburden testing to ensure product quality and safety.



















































![(a)
Fraction contaminated
= 2* (0.8/ 154)* (10/4*60)
= 0.00043
Contamination rate
= 1/ 0.00043
=2325.58
≈2326
(b)
Fraction contaminated
= 2* (0.8/ 154)*
[(1/60)/(4*60)]
= 7.215 * 10-7
Contamination rate
= 1/ 7.215 * 10-7
=1.38 * 108
≈ 1.4 * 108
69](https://image.slidesharecdn.com/bioburden-arpit-170317174824/85/Bioburden-69-320.jpg)



