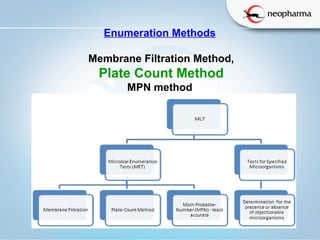
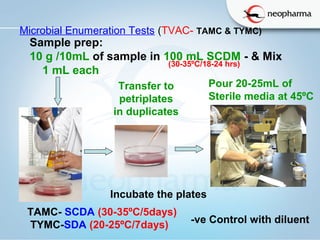
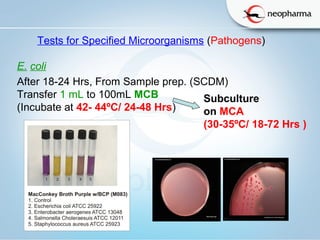
The document summarizes the harmonized microbial limit tests established in 2006 by the USP, EP, and JP pharmacopeias. The tests include microbial enumeration tests to determine total aerobic microbial count and total yeast and mold count, as well as tests for specified microorganisms like E. coli, Salmonella species, and Candida albicans. The tests involve preparing samples, incubating them in various growth media, and observing colonies to quantify microbes and identify pathogens based on standardized methods, limits, and interpretations. The harmonization aligned the structure, methods, and acceptance criteria used across different pharmacopeias to ensure microbial safety of non-sterile pharmaceutical products.