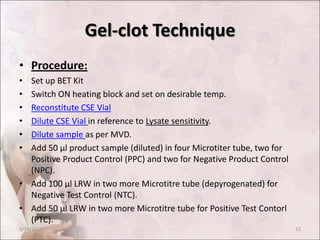
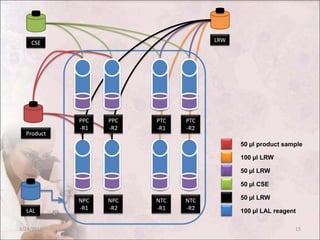
The document discusses the bacterial endotoxin test, which is used to detect or quantify endotoxins from gram-negative bacteria. It defines endotoxins as lipopolysaccharides found in the outer membrane of gram-negative bacteria that are released upon cell lysis. The test utilizes a gel clot formation reaction that occurs when endotoxins interact with amoebocyte lysate from the horseshoe crab. The document outlines the principle, materials, procedures, controls, and interpretation for the gel clot technique of performing the bacterial endotoxin test on pharmaceutical products to ensure quality standards are met.