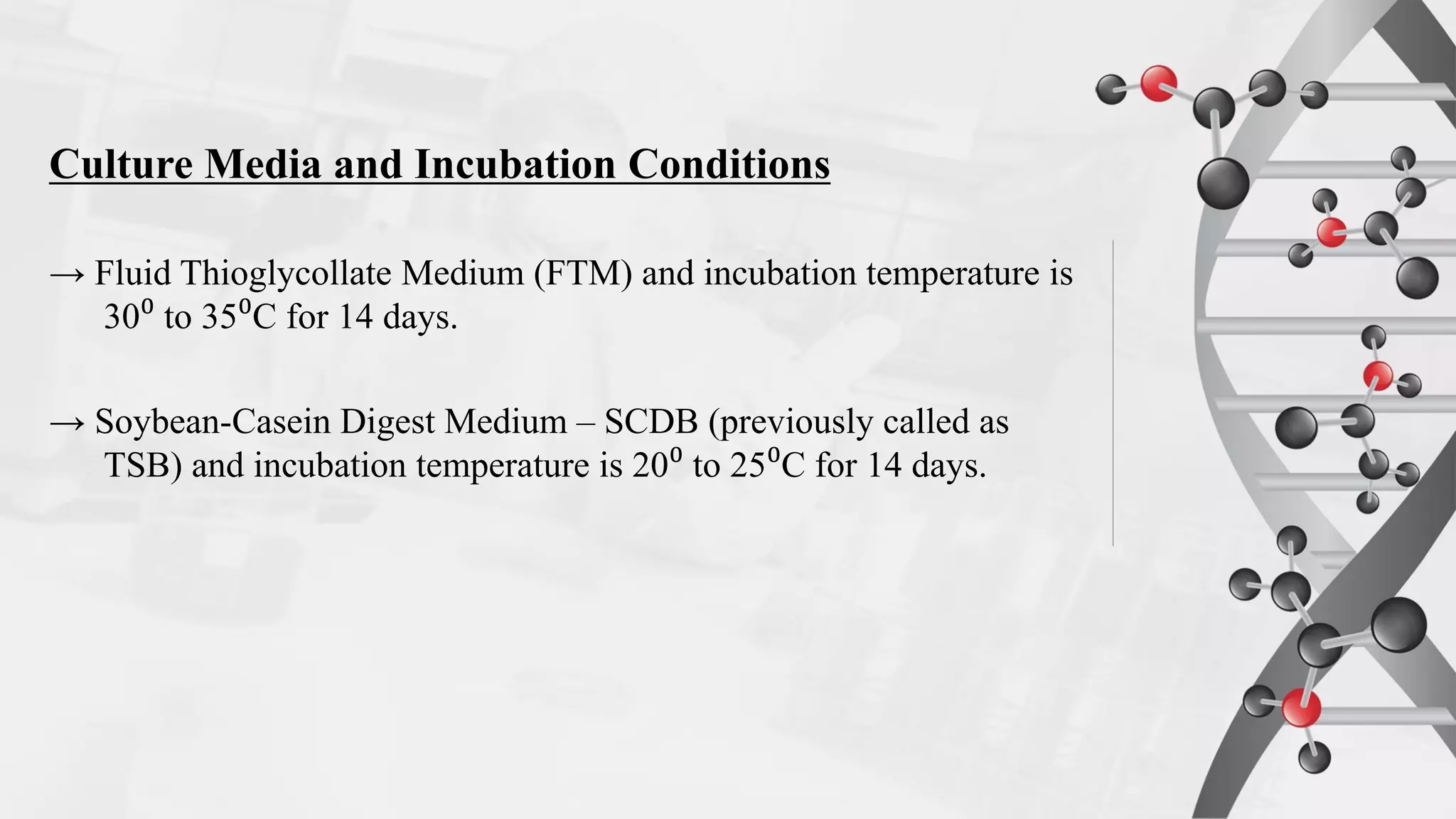
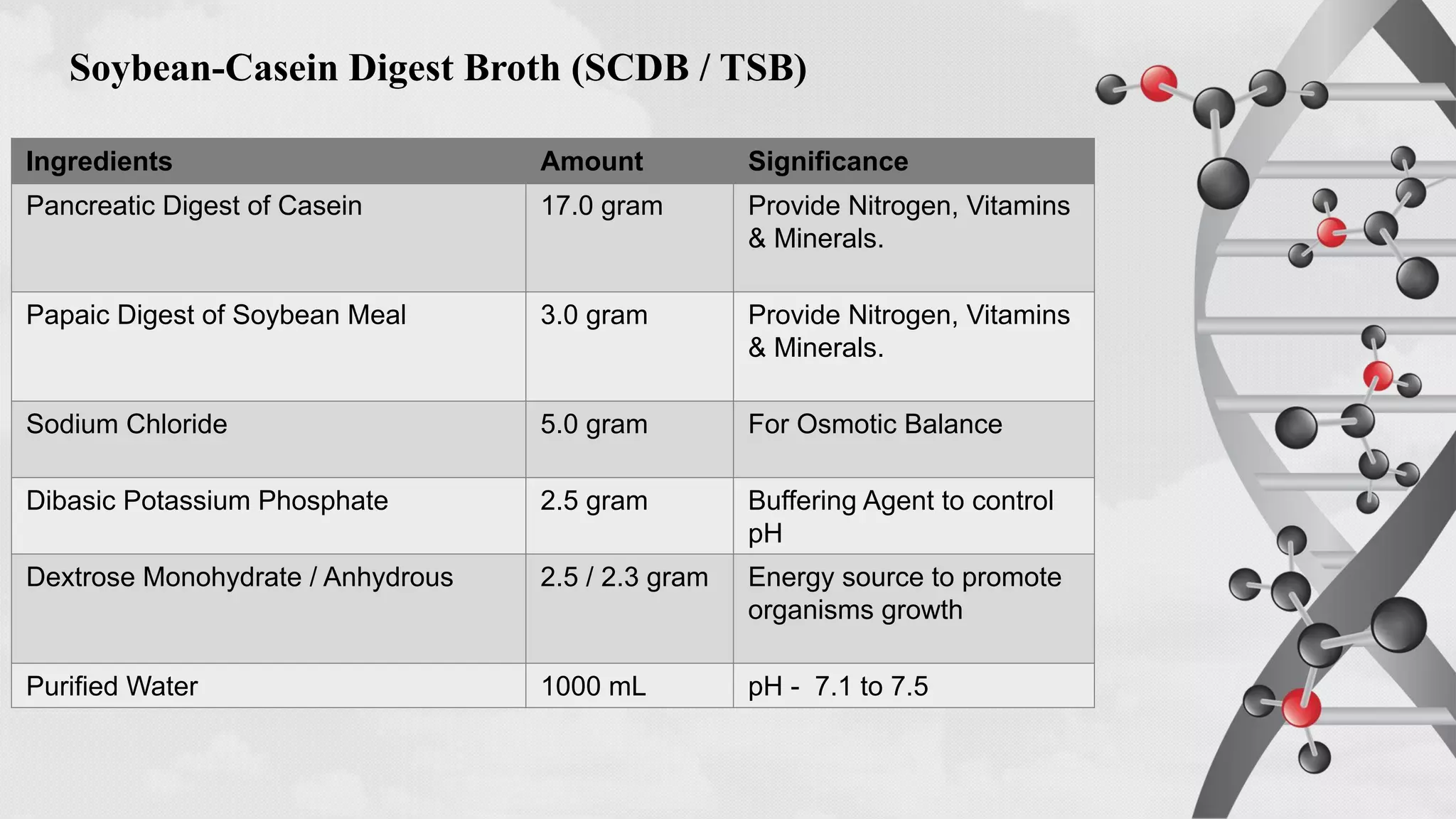
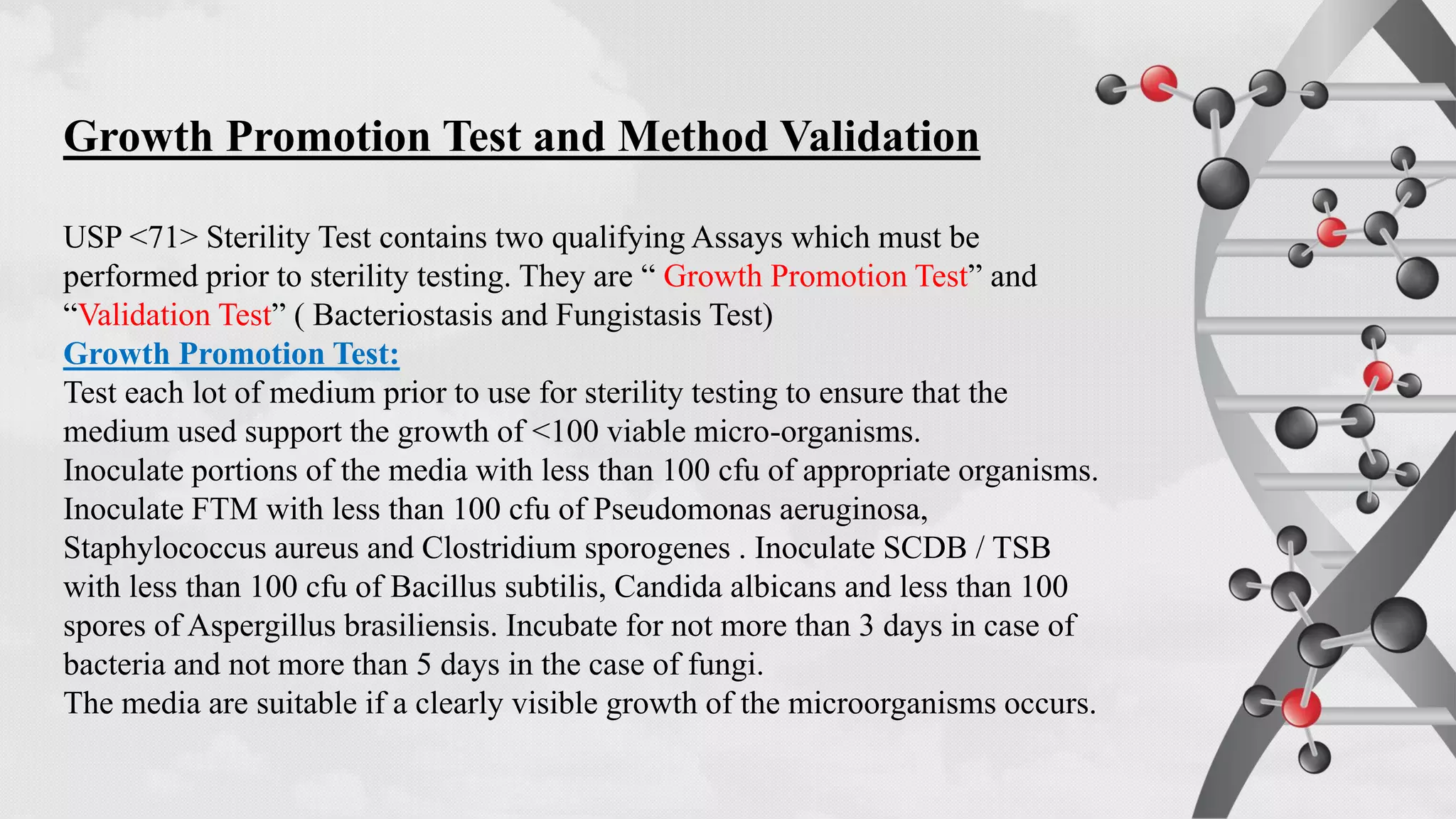
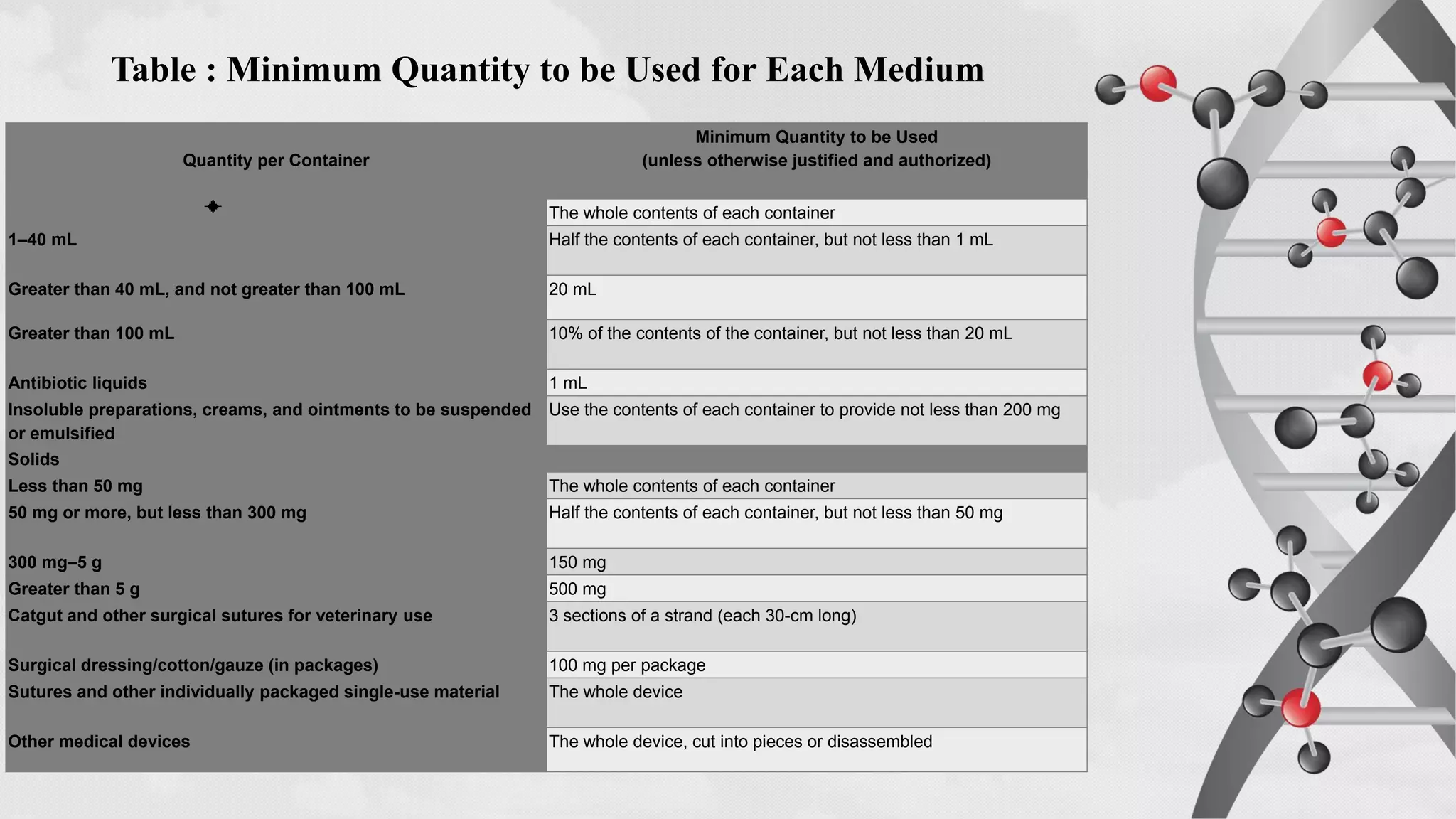
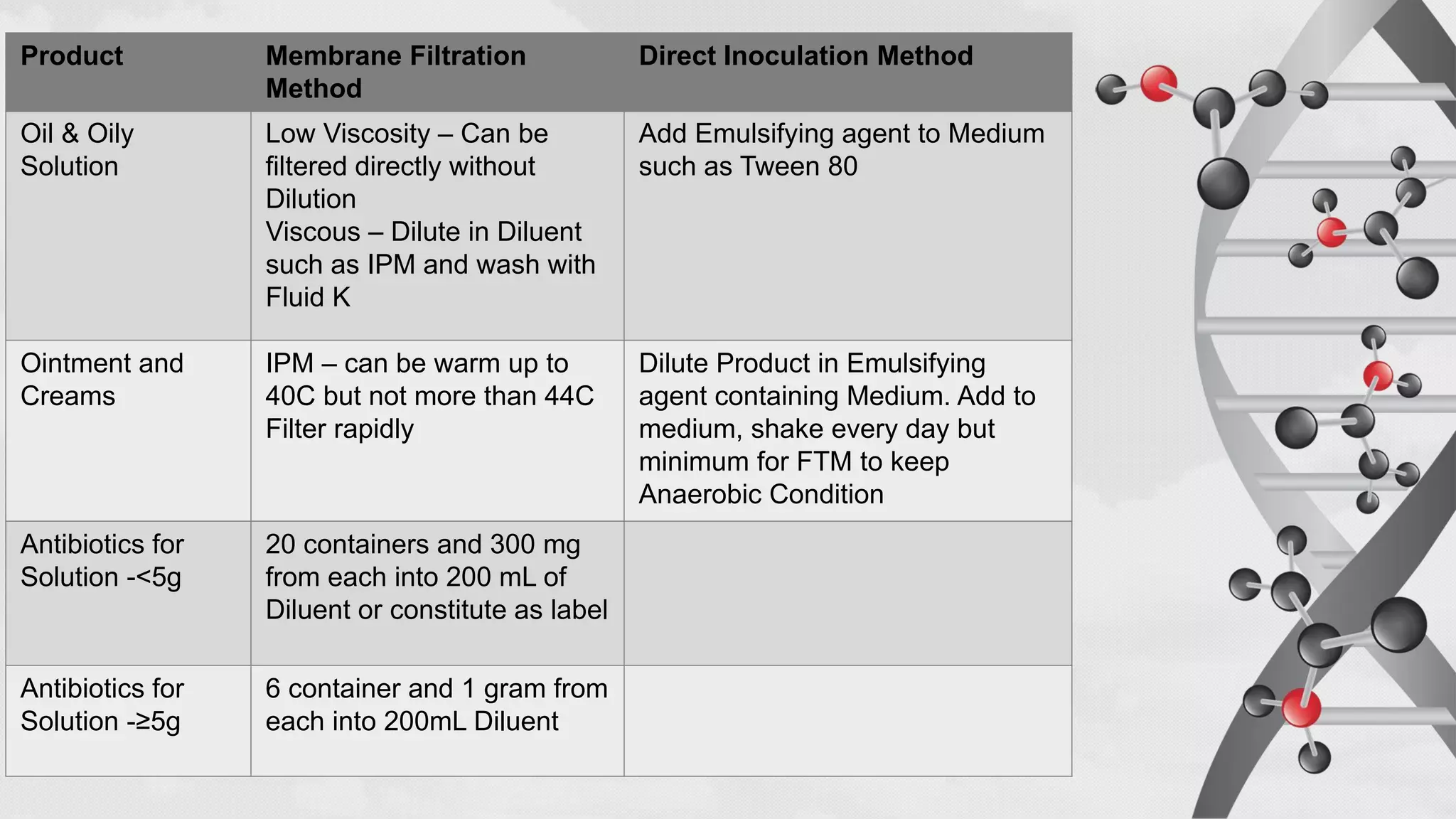
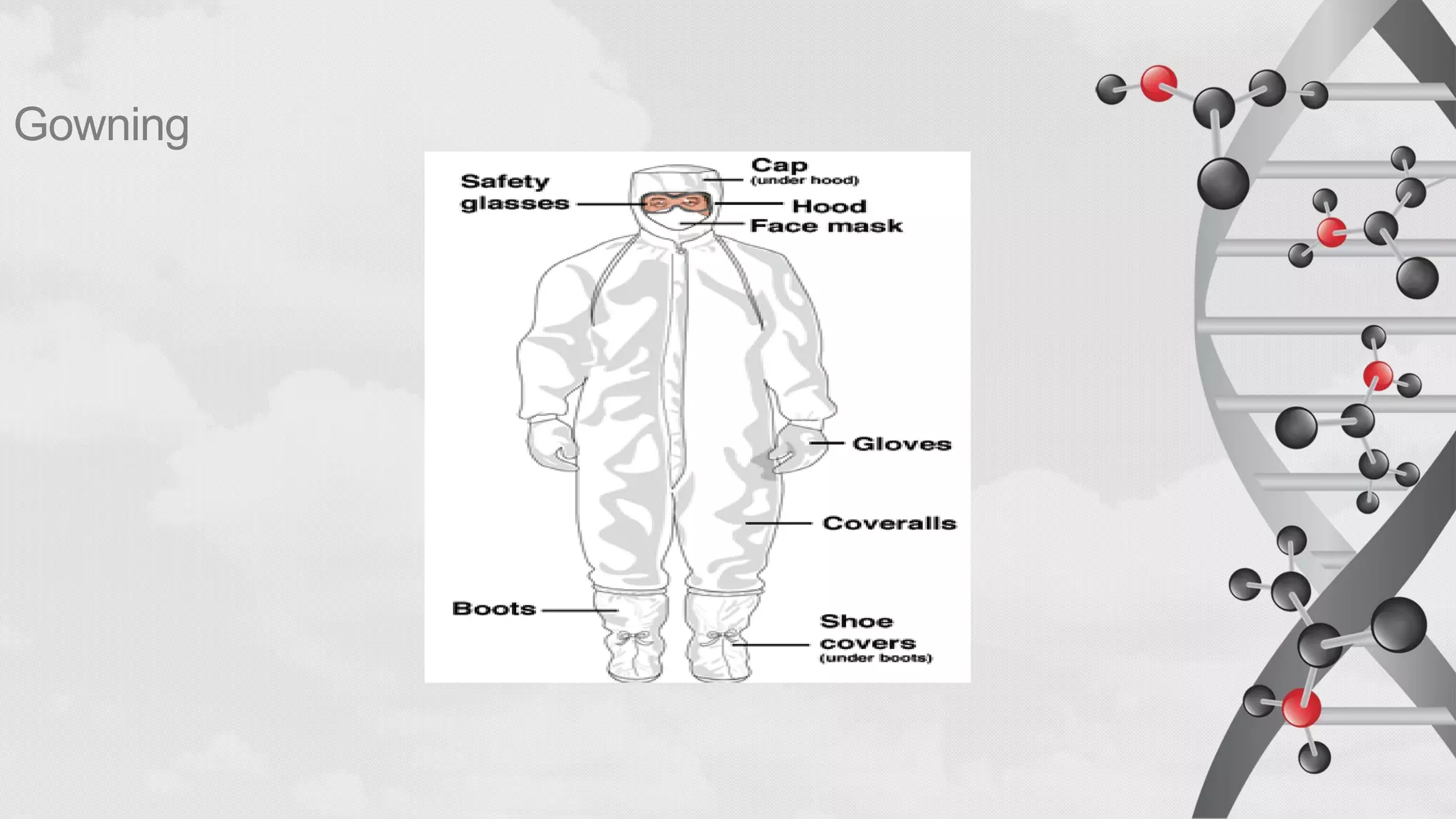
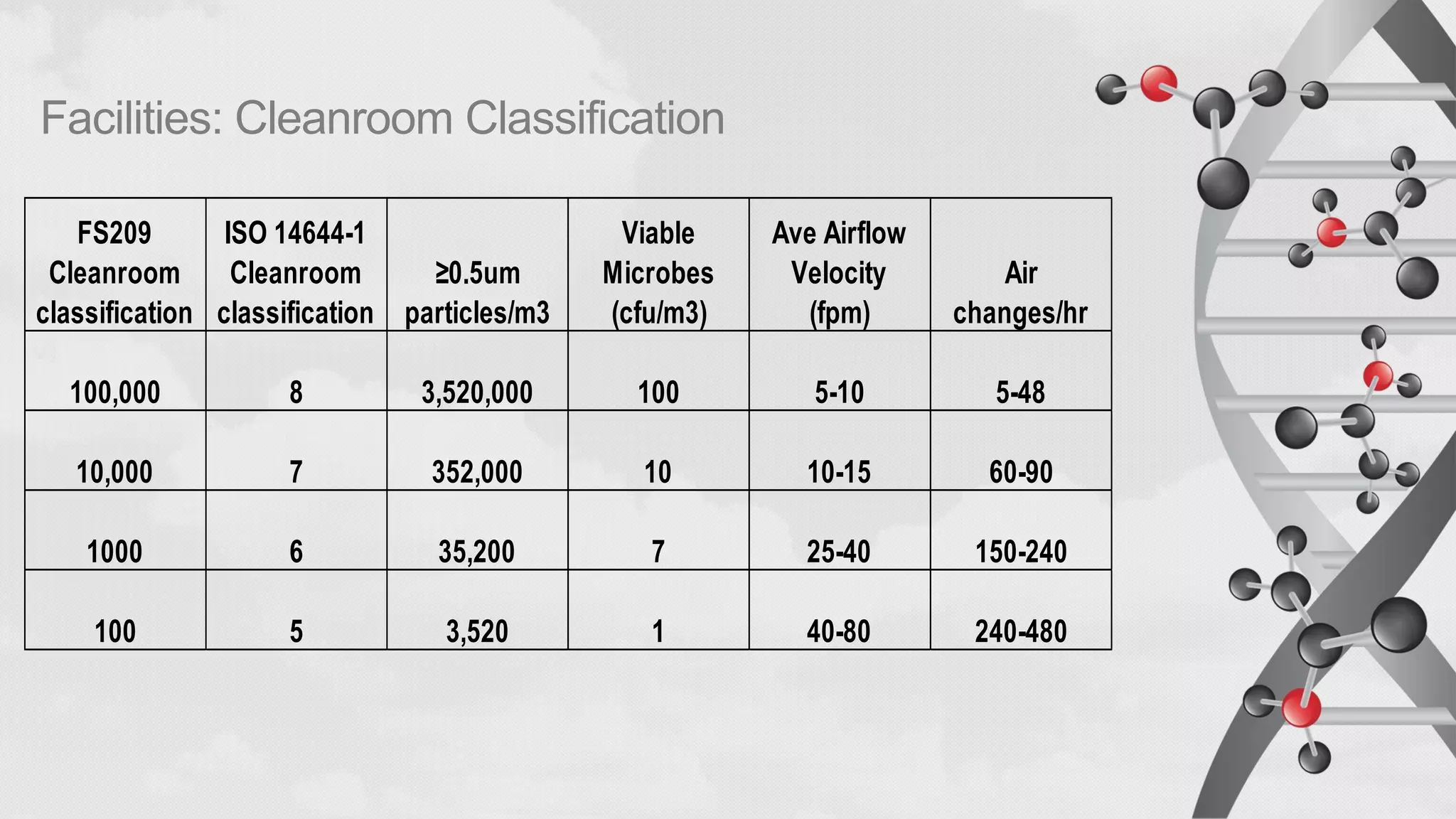
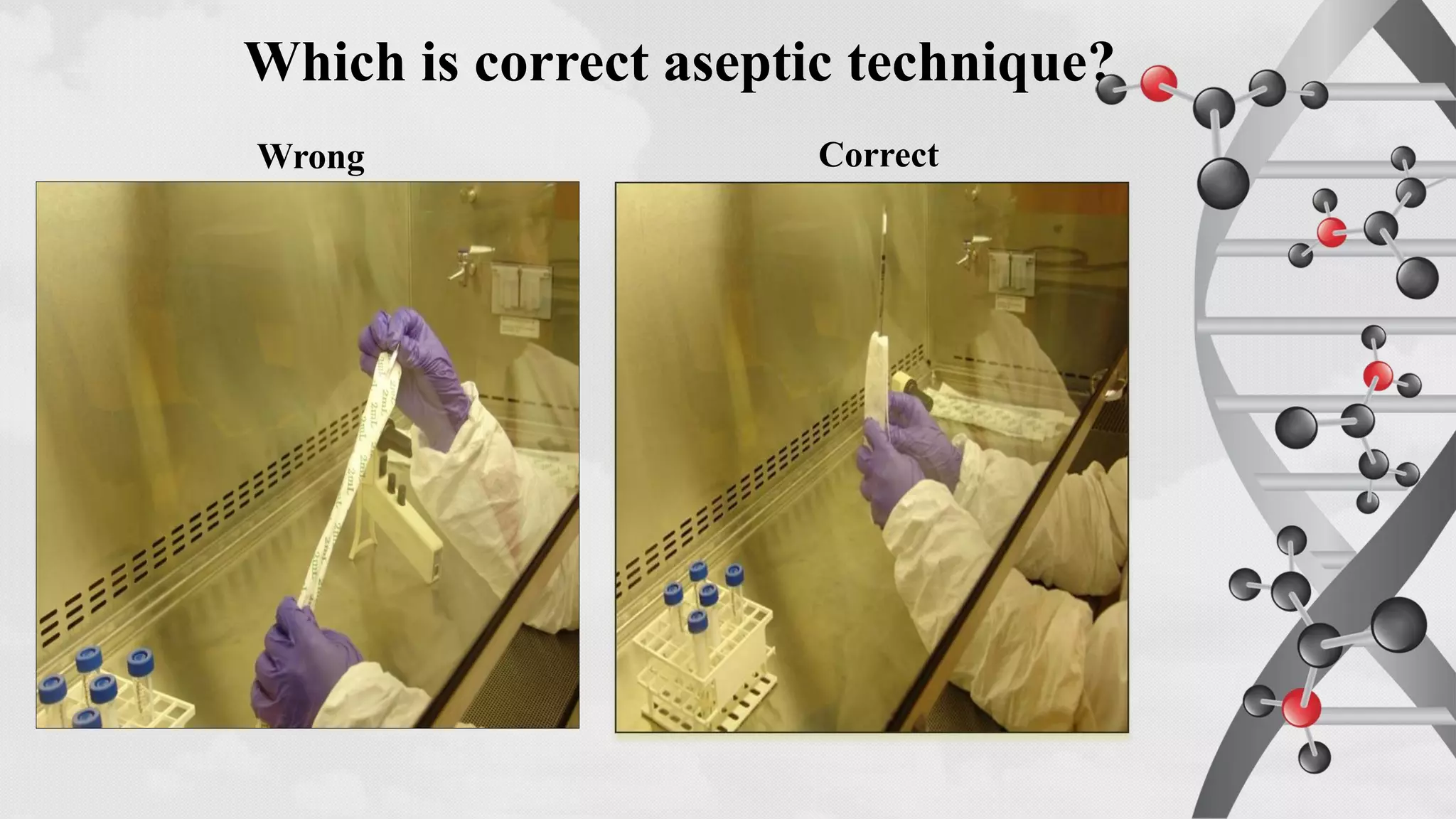
The document provides comprehensive guidelines on sterility testing, highlighting its importance in ensuring medical products are free from viable microorganisms. It details specific procedures, including required culture media, incubation conditions, and testing methods such as membrane filtration and direct inoculation. Additionally, it emphasizes the significance of method validation, personnel training, and environmental controls to maintain sterile conditions during testing.