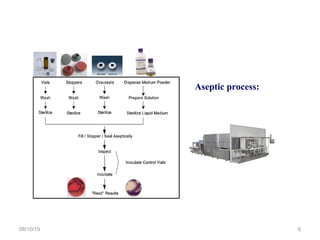
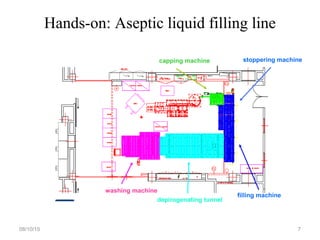
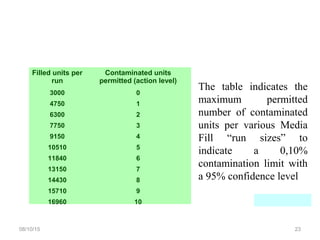
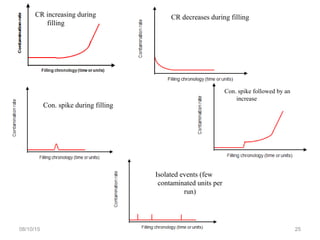
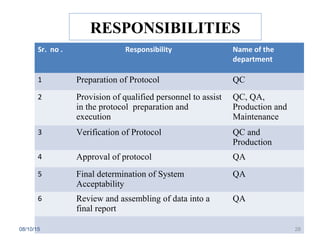
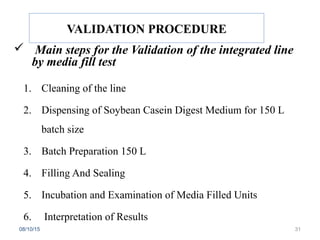
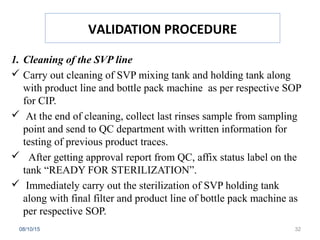
The document outlines the media fill process and its validation, which simulates normal manufacturing operations for microbiological growth media to ensure aseptic conditions. It emphasizes the importance of validating aseptic processes as per pharmaceutical regulations to minimize contamination risks and details various protocol aspects, including frequency of runs, medium culture requirements, container considerations, and monitoring activities. Additionally, it specifies acceptance criteria for contamination rates and corrective actions in case of failures.