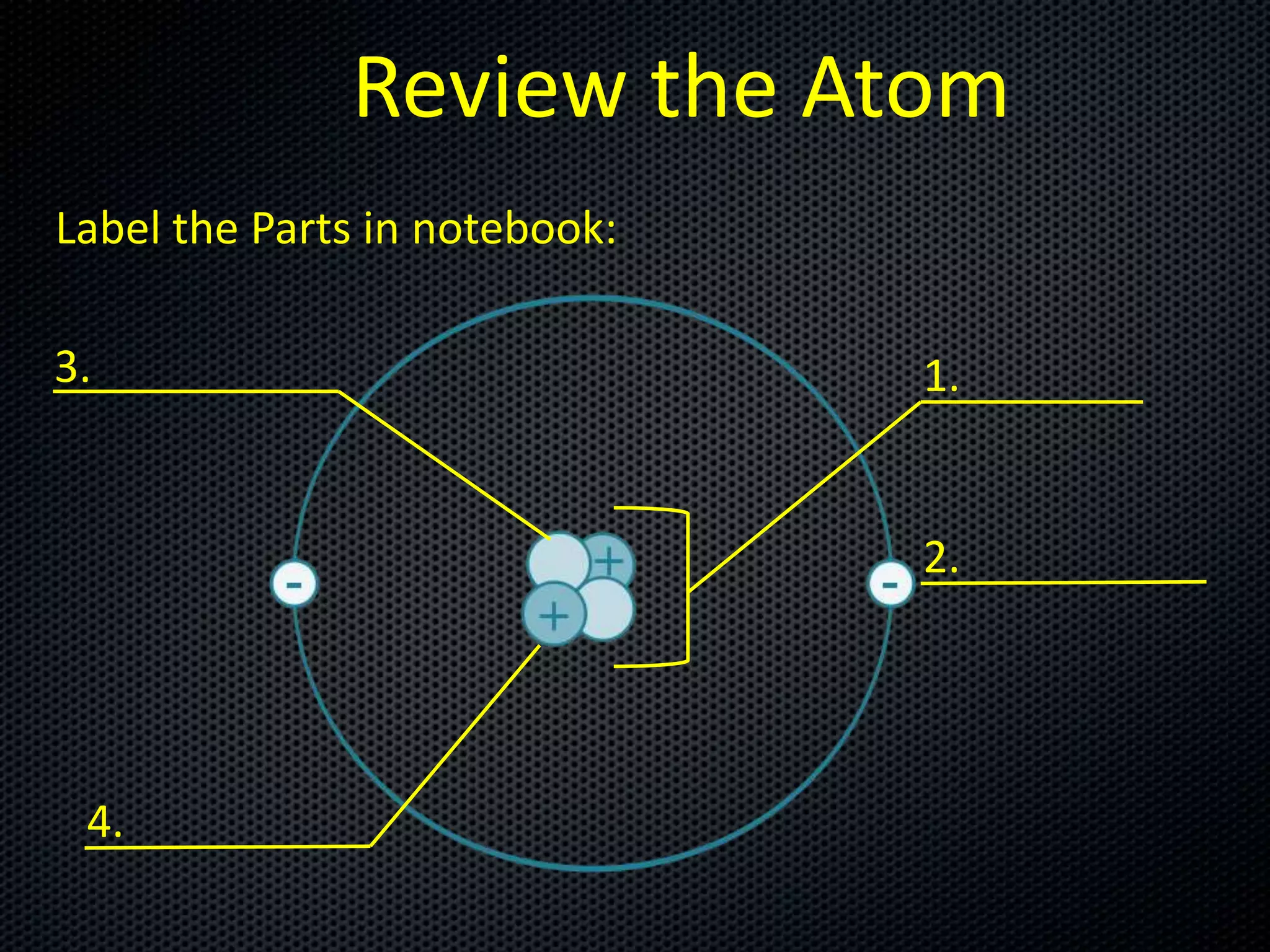
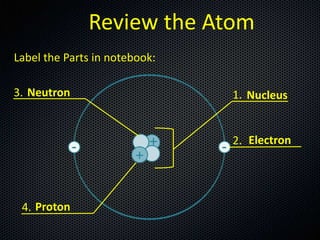
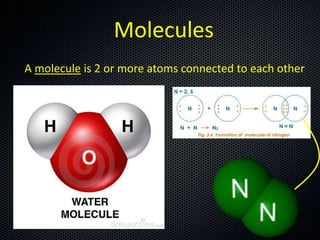
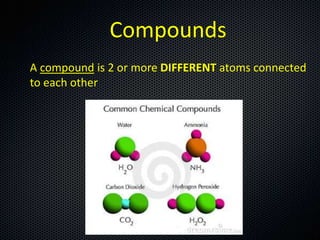
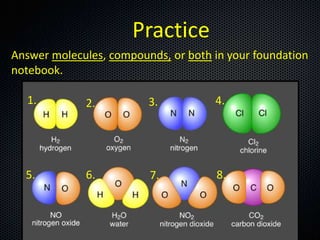
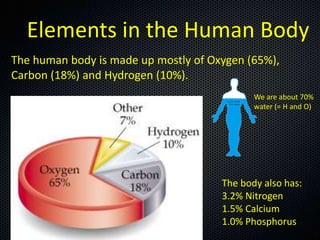
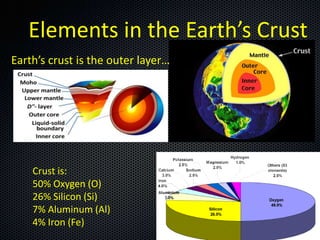
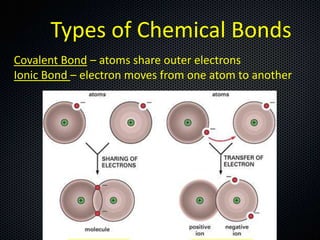
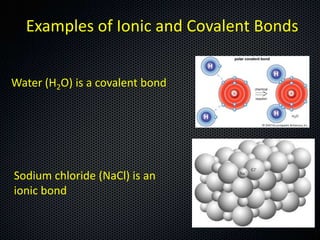
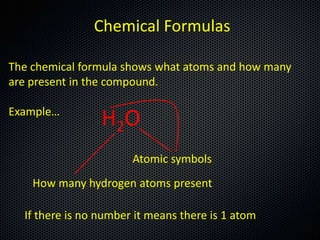
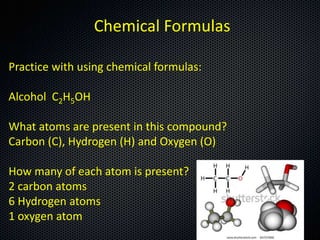
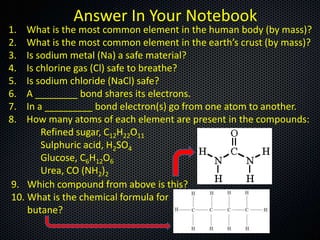
The document discusses elements, compounds, molecules, and chemical bonds. It provides examples of ionic bonds (sodium chloride) and covalent bonds (water). It defines a molecule as two or more atoms connected together, and a compound as two or more different atoms connected together. The document also discusses the chemical formulas of compounds like alcohol and glucose, showing the atoms present and their quantities.