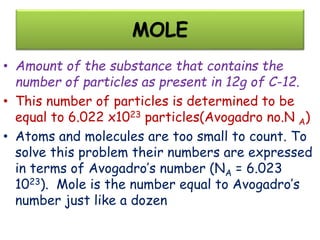
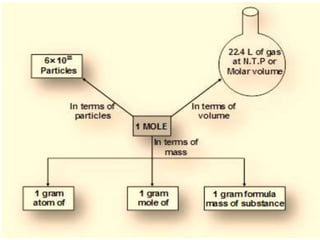
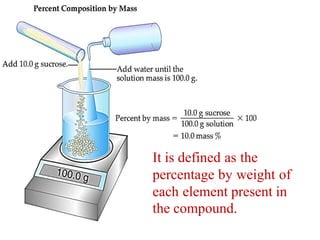
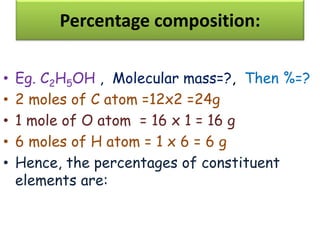
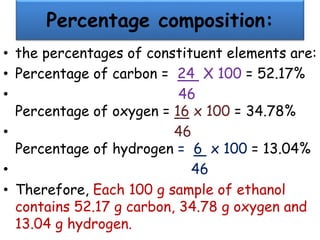
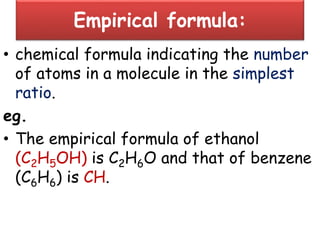
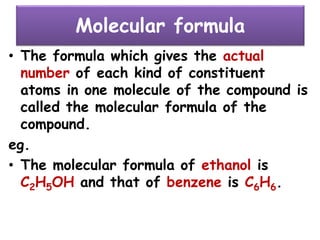
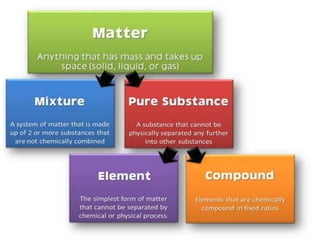
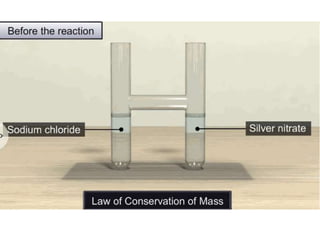
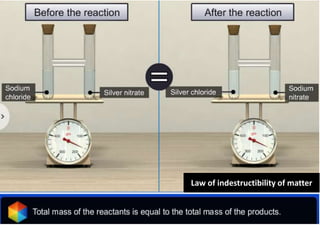
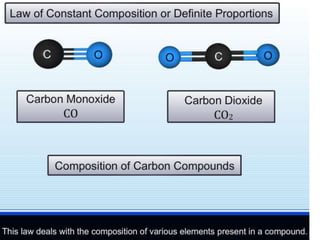
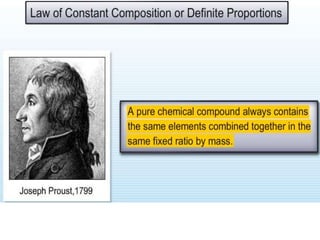
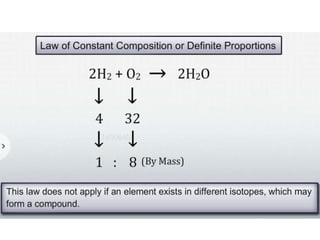
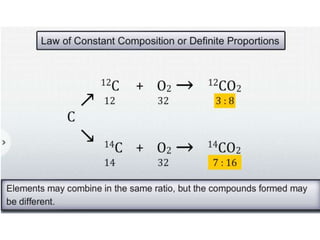
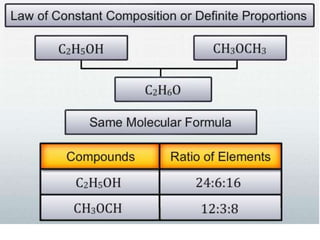
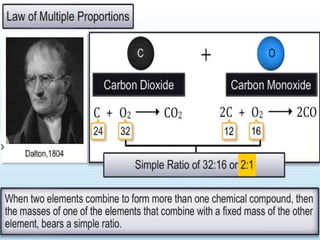
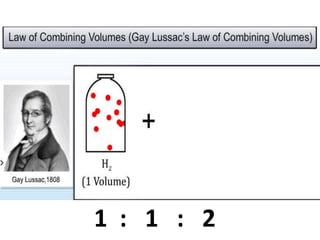
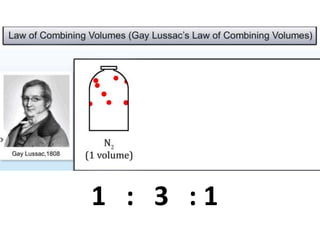
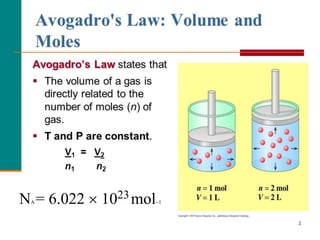
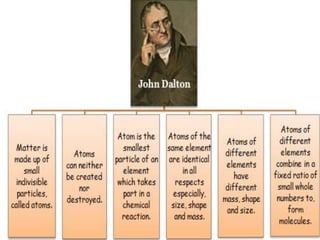
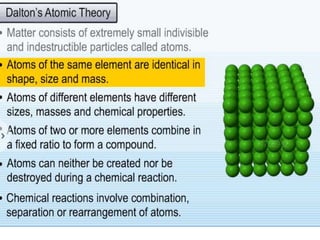
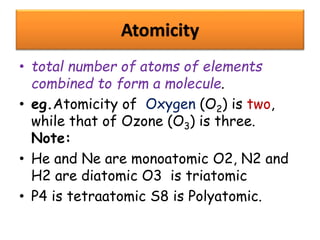
Chemistry is the study of matter and its interactions. The basic units that make up all matter are atoms, which combine to form molecules. Atoms are made up of protons, neutrons, and electrons. The number of protons in an atom determines its atomic number, while the total mass of an atom is determined by its atomic mass number. Molecules are combinations of atoms that are bonded together chemically. The mole is a unit used to measure amounts of substances, equal to 6.022x1023 particles. Stoichiometry uses mole ratios to calculate amounts of reactants and products in chemical reactions.



















![Atom is distinguished from its molecule
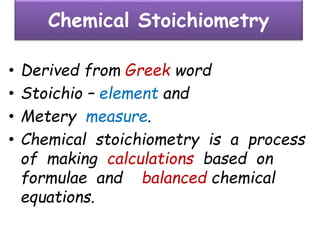
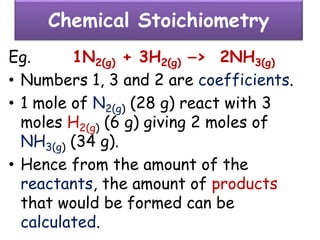
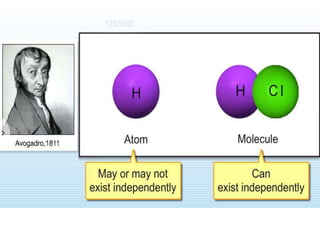
• Consider the formation of hydrogen chloride from
hydrogen and chlorine.
• Hydrogen and chlorine do not exist in free atomic
state but exist in molecular state hydrogen chloride.
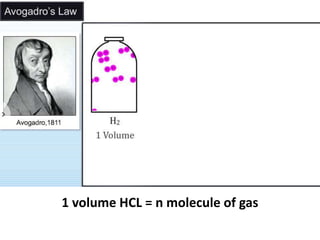
H2 + Cl2 --> 2HCl
[1v] [1v] [2 volumes]
[n molecules] [n molecules] [2n molecules]
½ ½ 1](https://image.slidesharecdn.com/somebasicconceptofchemistry2017-180302121024/85/Some-basic-concept-of-chemistry-2017-63-320.jpg)