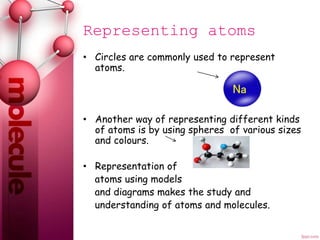
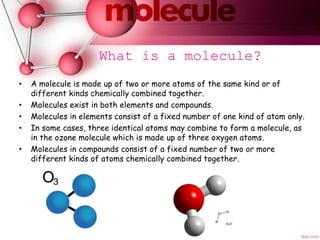
An atom is the smallest particle of an element that can exist. Atoms combine to form molecules, which are the basic units of compounds and some elements. Molecules can be represented by chemical formulas that show the number and type of atoms in the molecule, such as H2O for a water molecule containing two hydrogen atoms and one oxygen atom. Circles and spheres are also used to model atoms and molecules to aid in the study of their structures.