Embed presentation
Downloaded 107 times

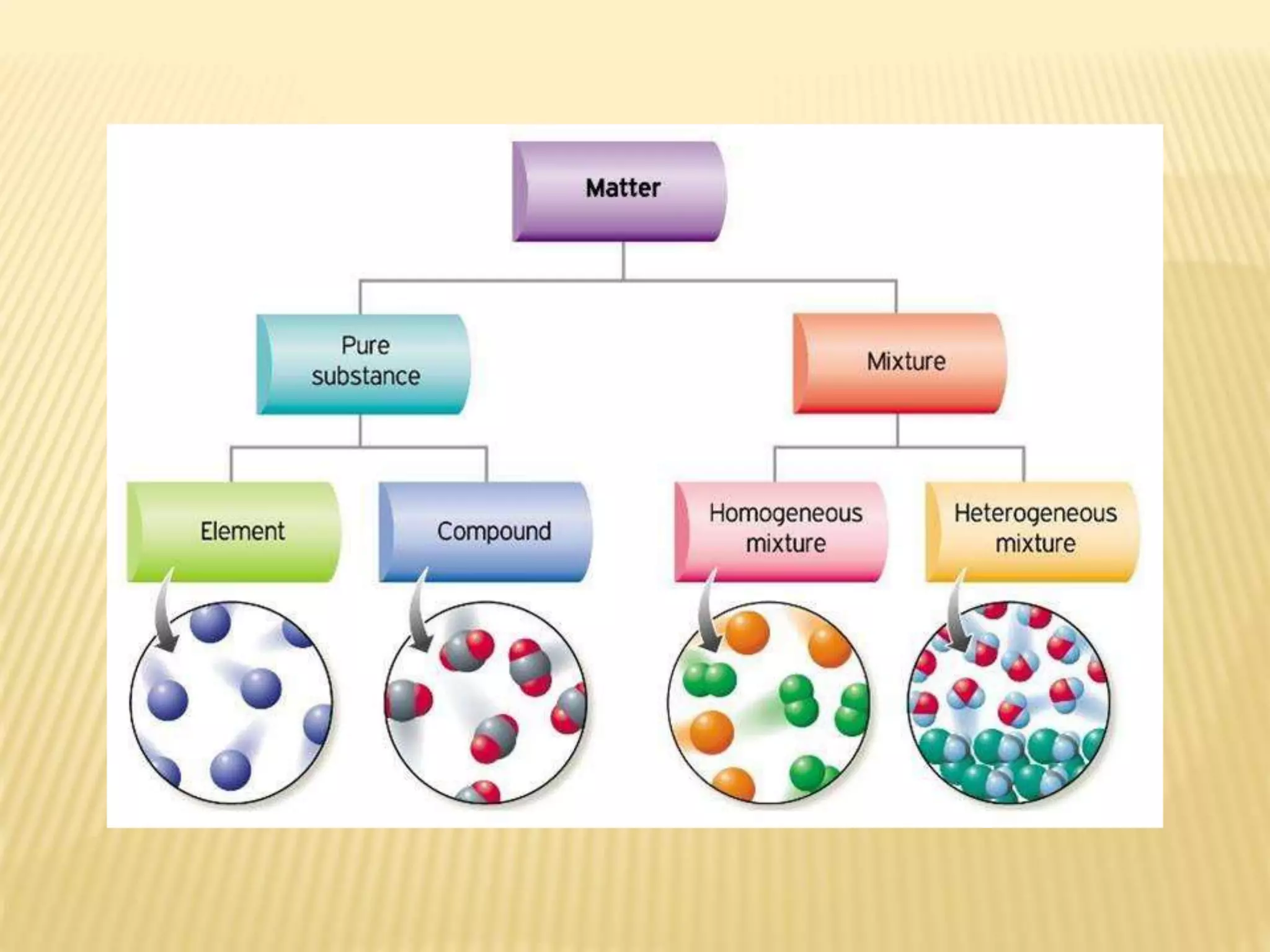


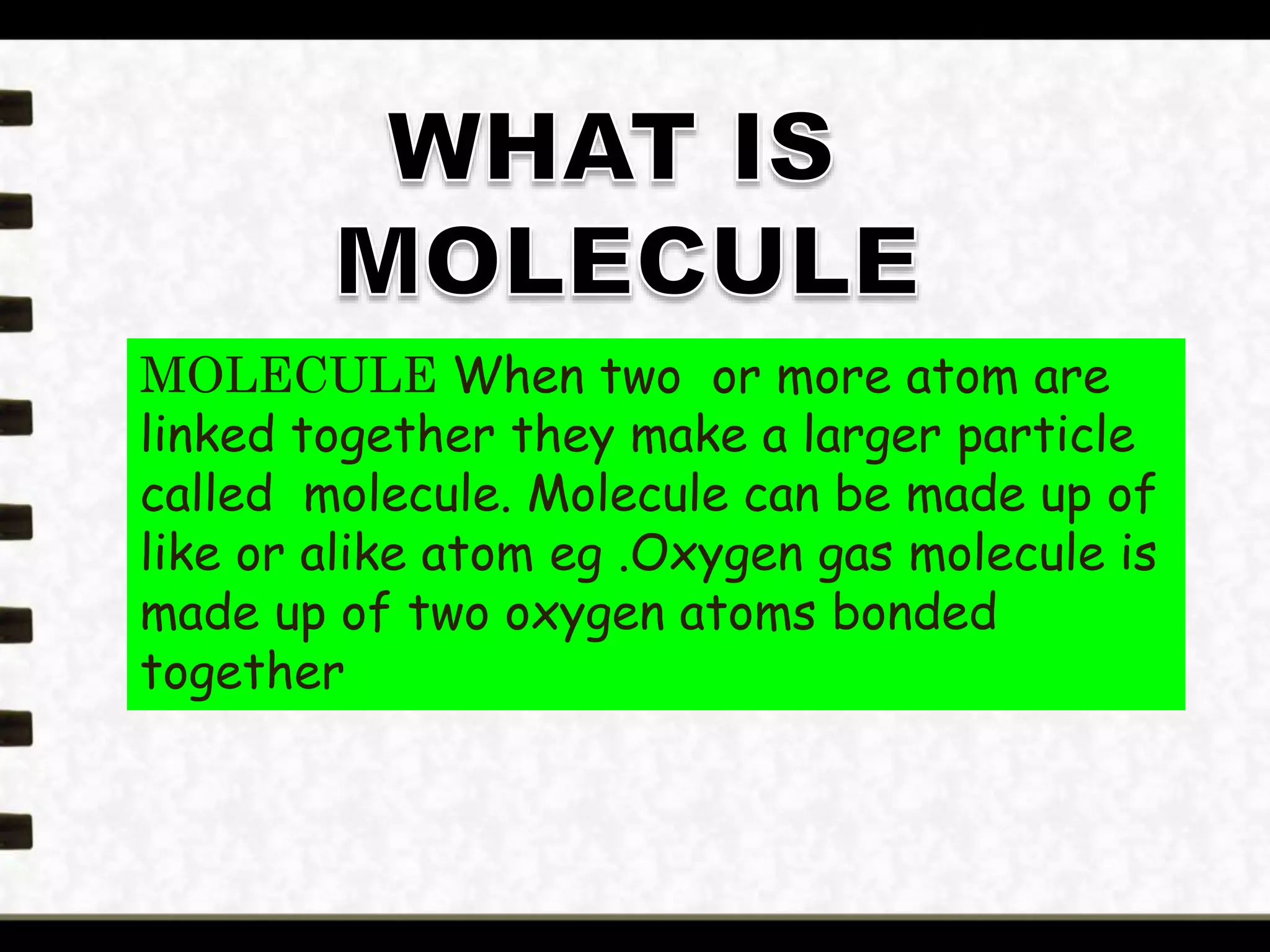

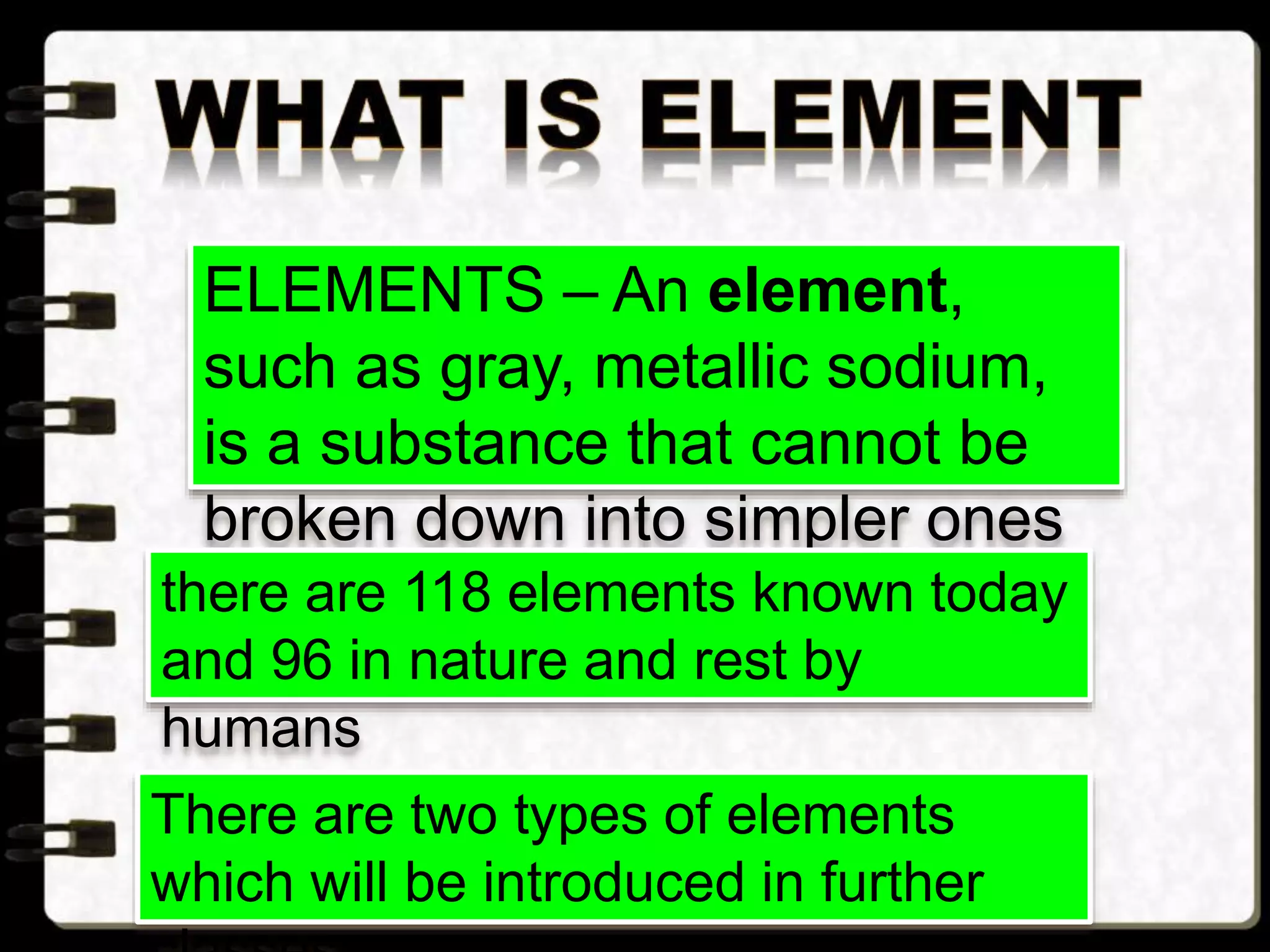

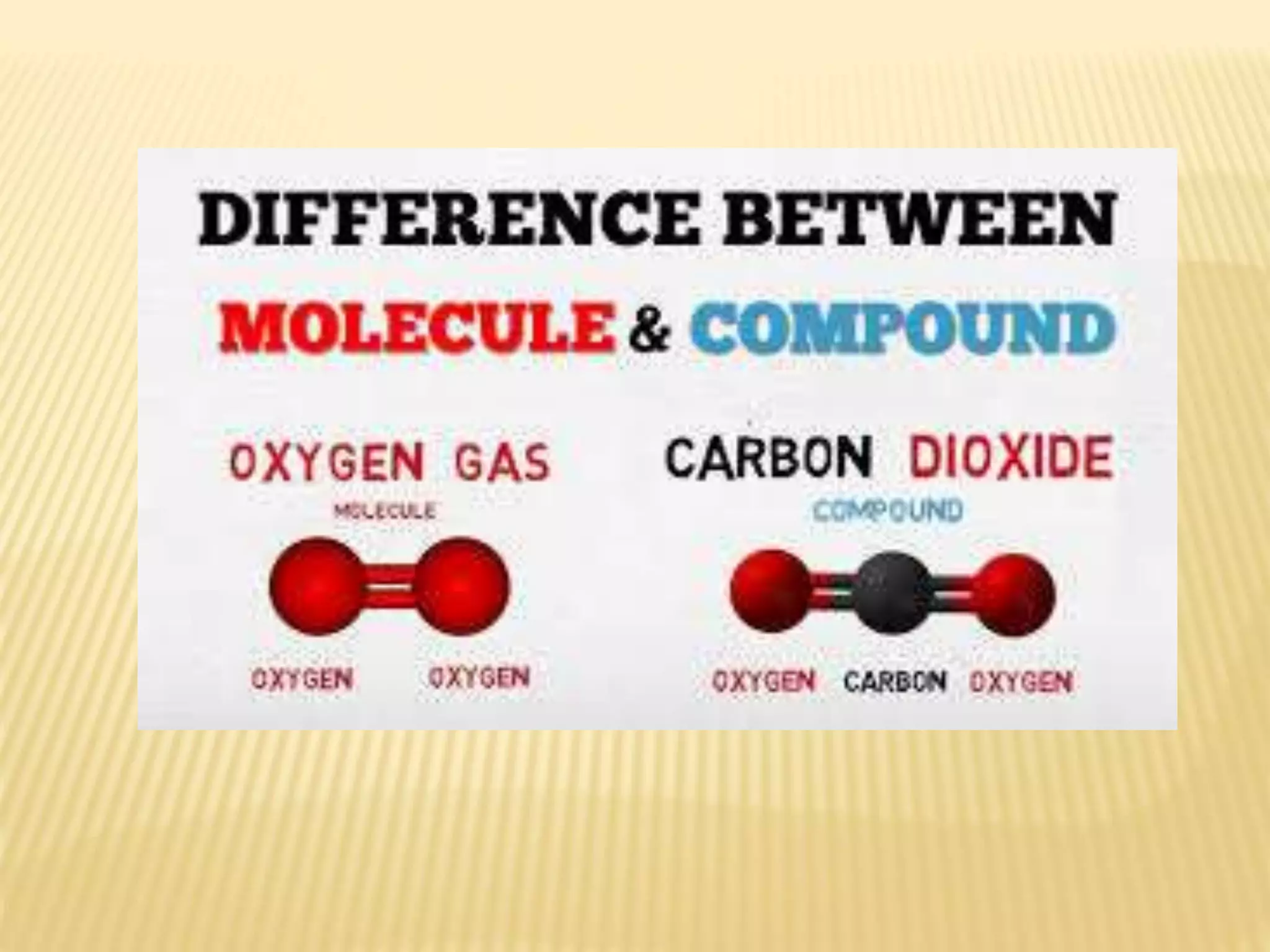
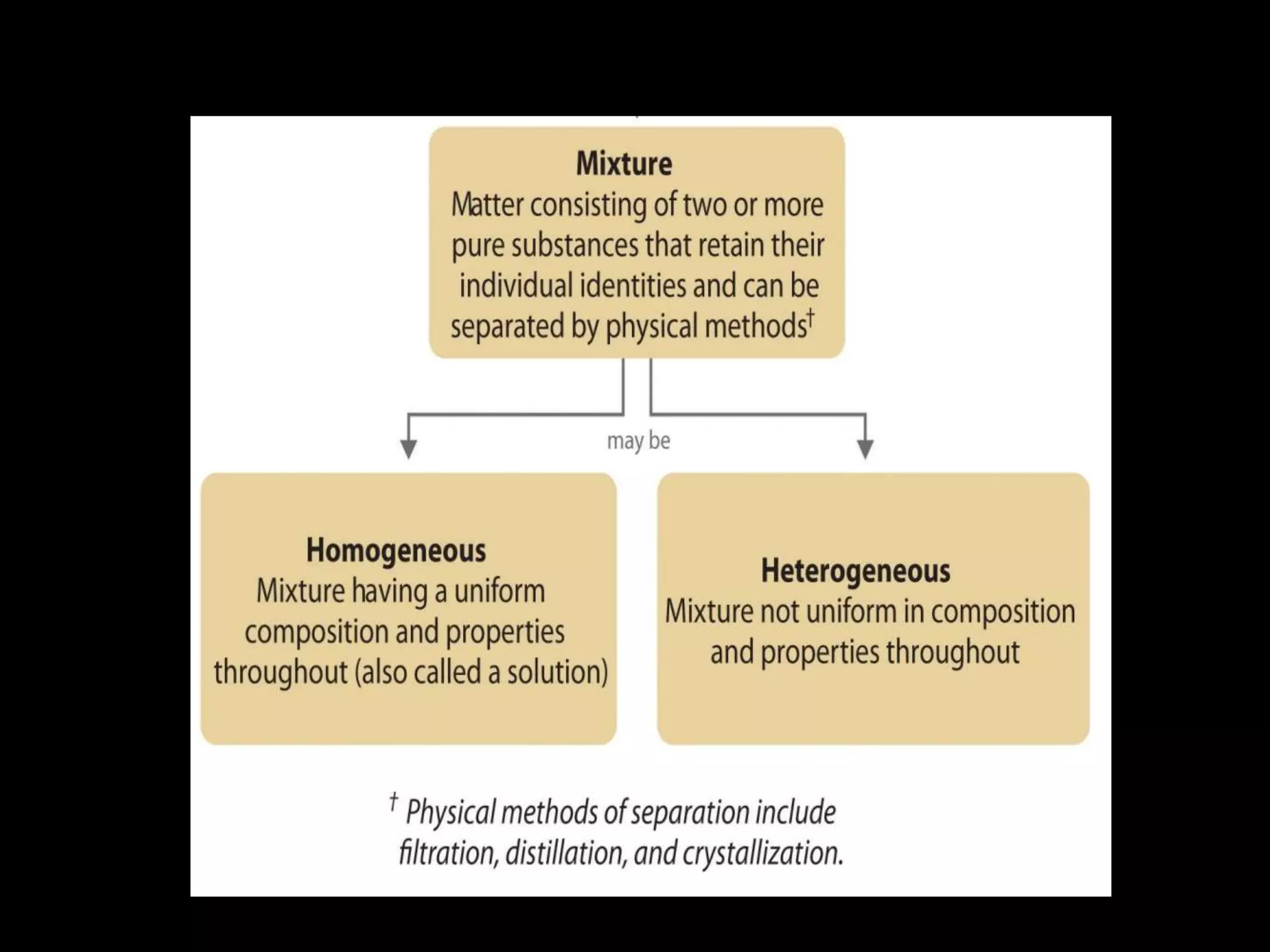
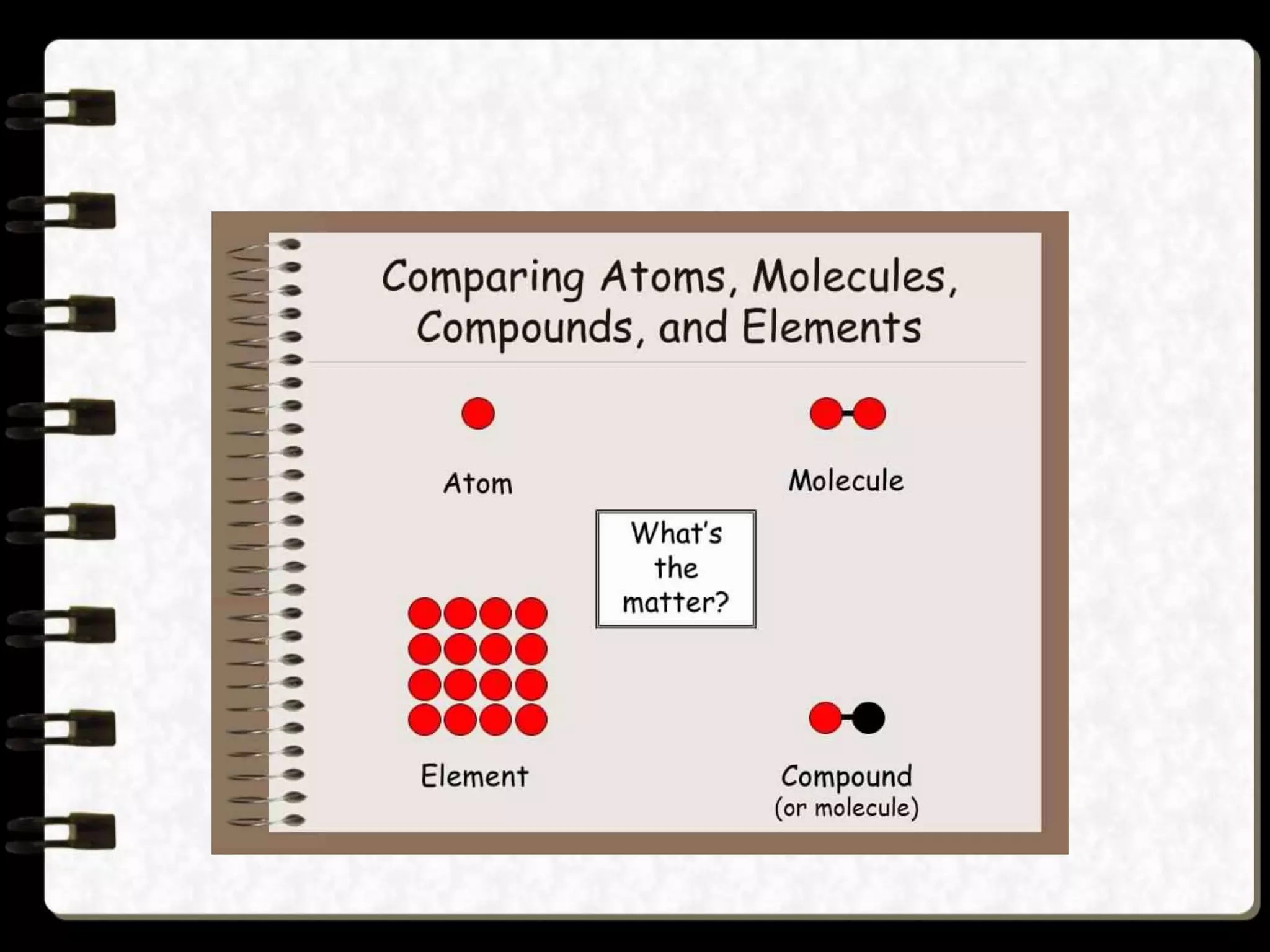


Matter is made up of tiny particles called atoms. Atoms are the basic unit of matter. When two or more atoms are linked together they form molecules, which can be made up of identical or different types of atoms. Elements are substances that cannot be broken down into simpler substances by chemical changes, and there are currently 118 known elements. Compounds are pure substances made up of two or more elements combined together in fixed proportions that can be decomposed into their constituent elements.











