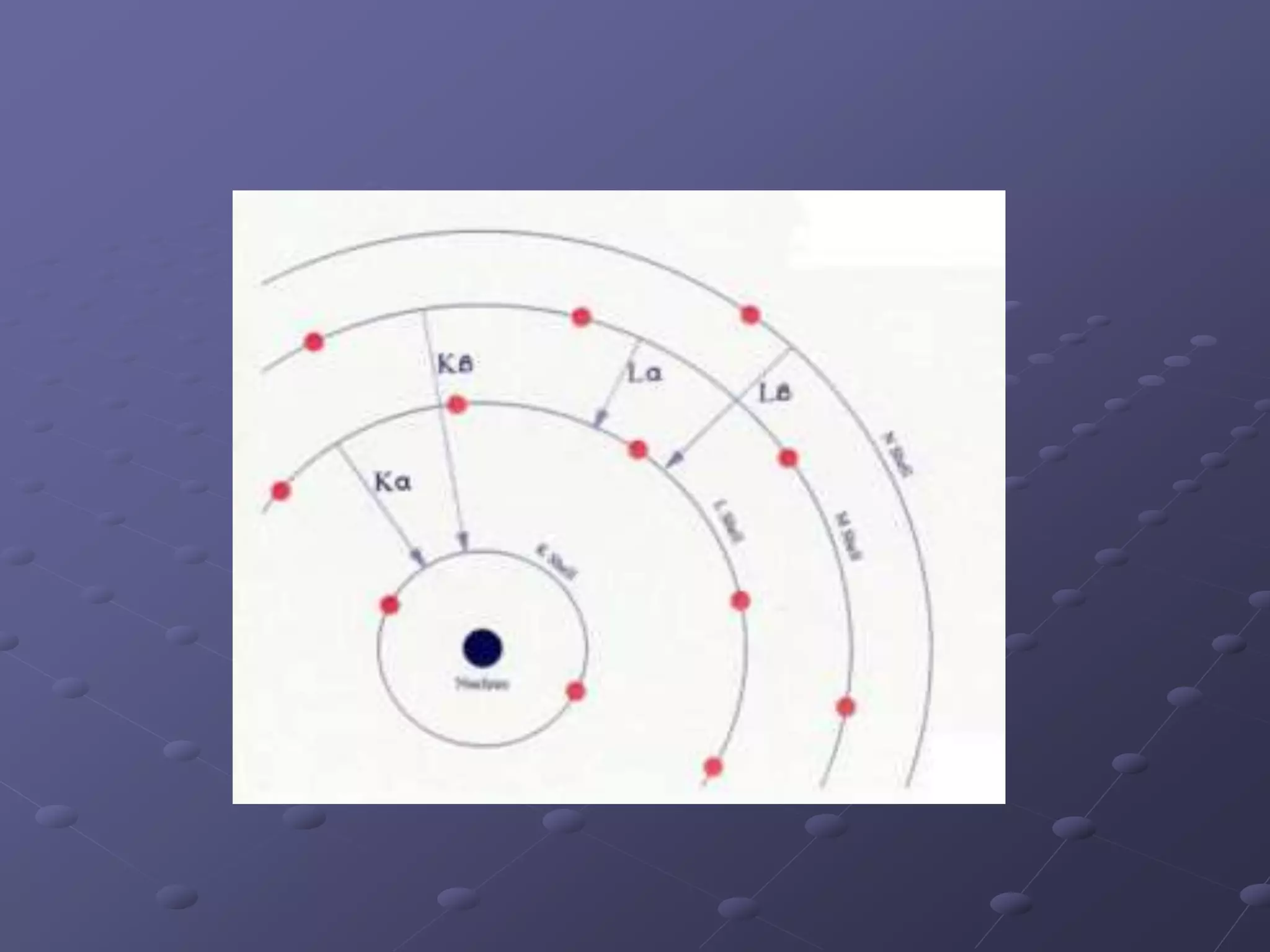
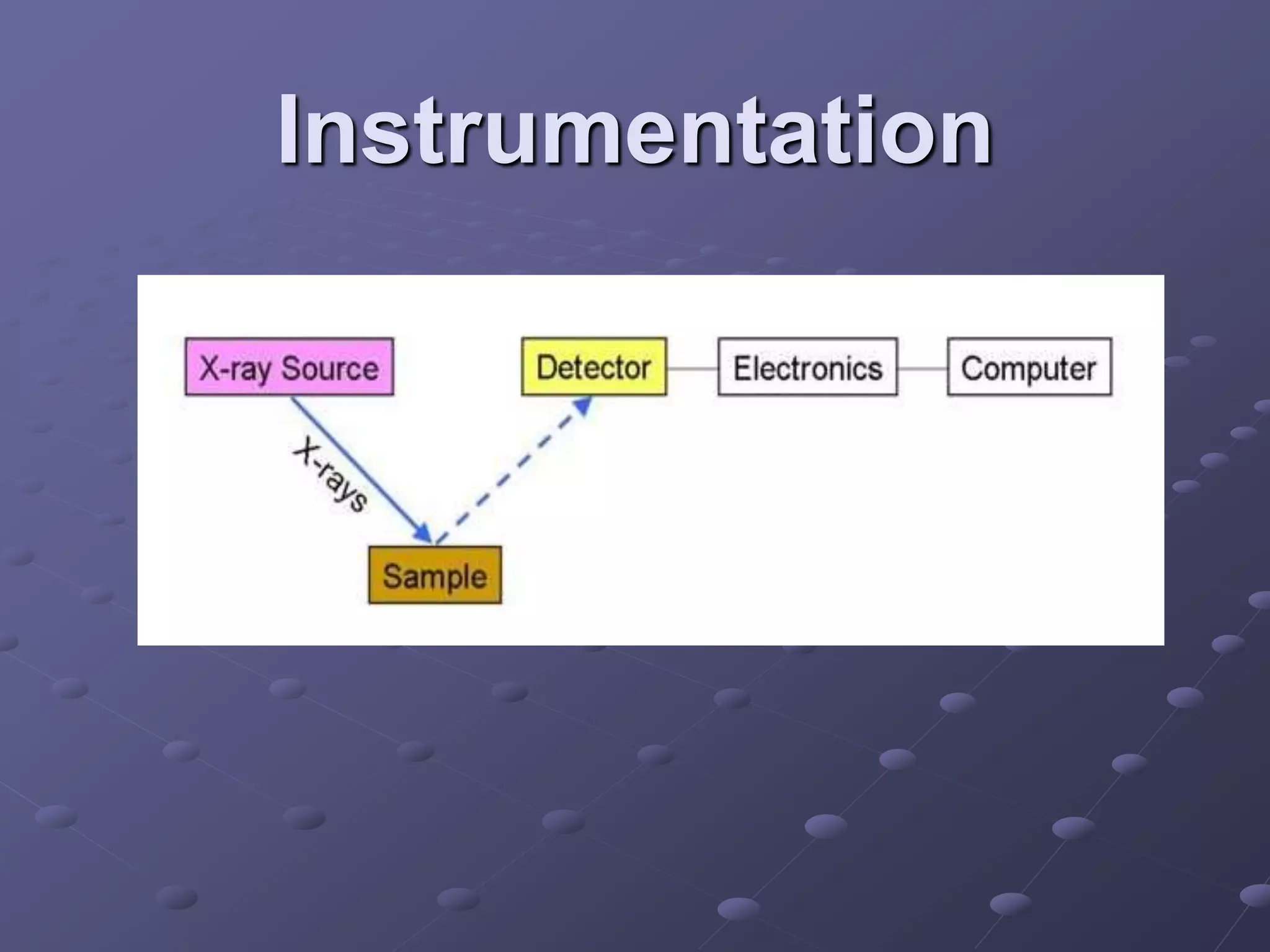
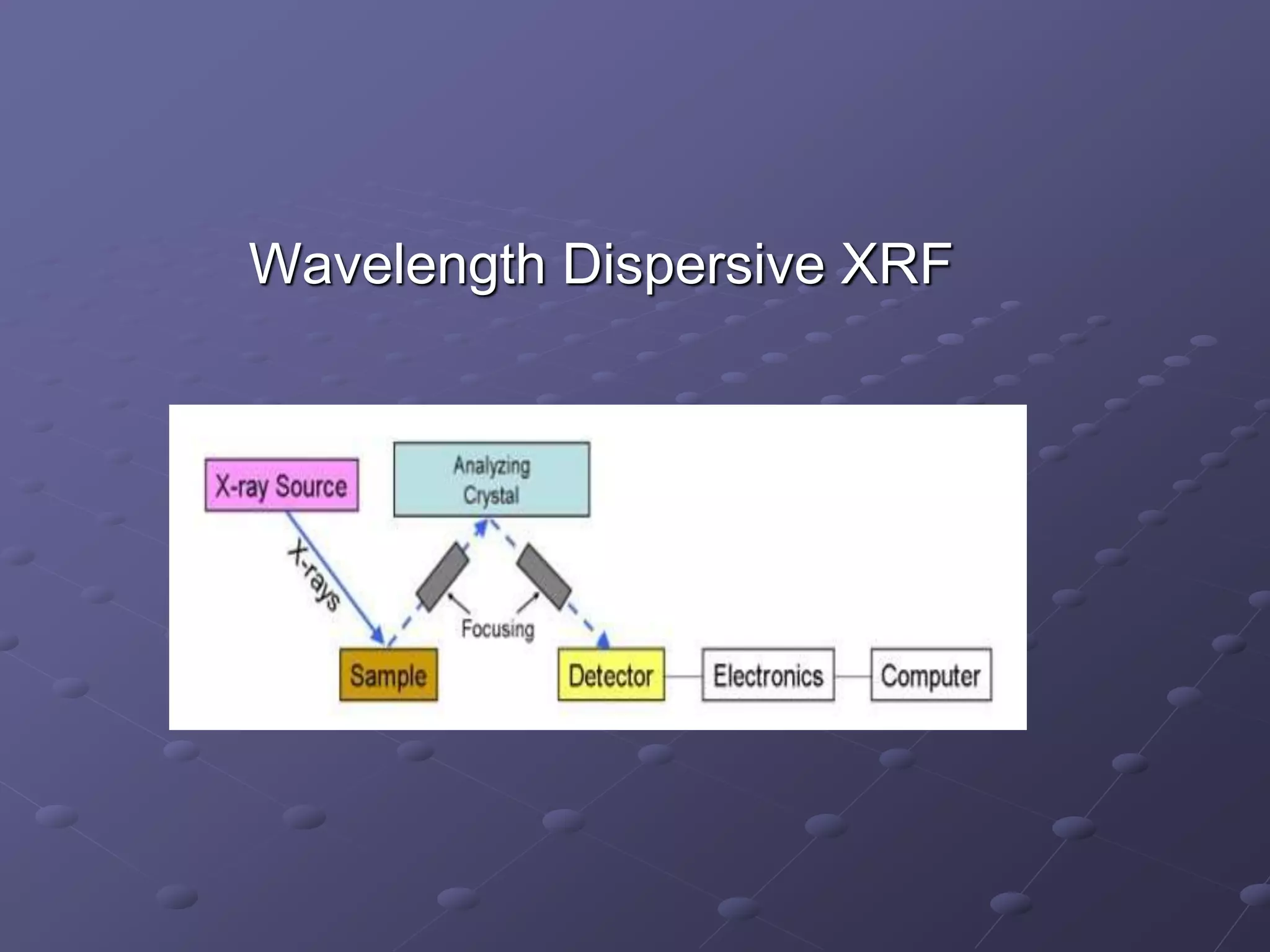
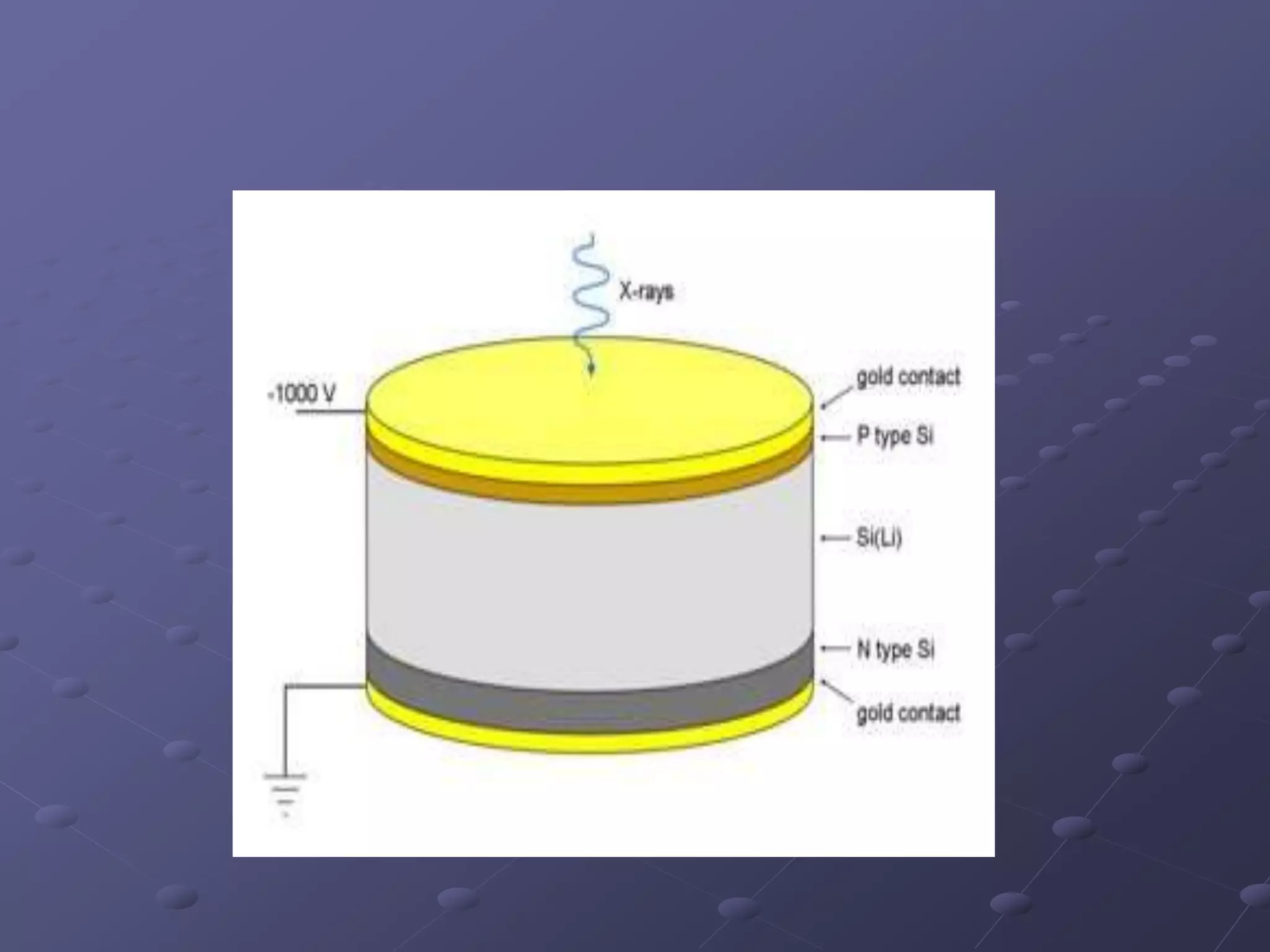
X-ray fluorescence is a technique used to analyze the elemental composition of materials. It works by using X-rays to excite electrons in the inner shells of atoms within a sample. This causes the emission of characteristic X-rays from the outer electron shells filling the inner shell vacancies. The energies of these emitted X-rays are analyzed to identify the elemental composition of the sample. Common applications of this technique include analysis of materials in forensic investigations and archaeological specimens to determine their elemental makeup.