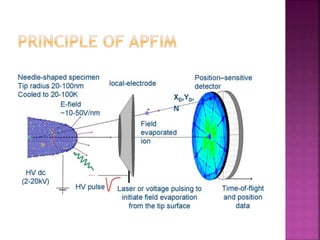
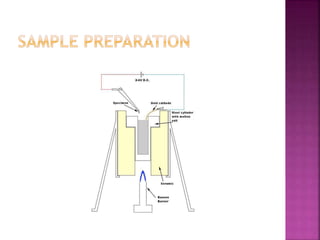
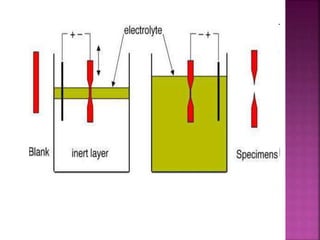
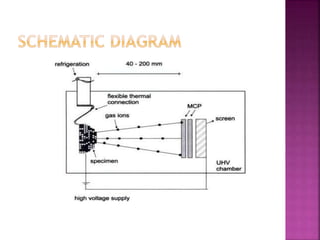
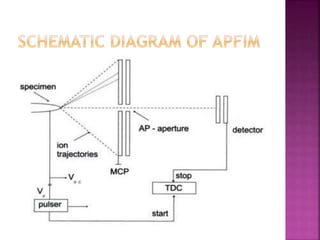
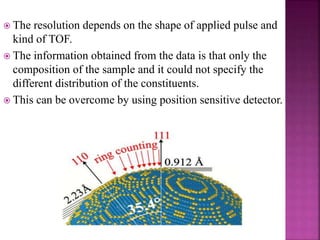
The document discusses atom probe field ion microscopy, a technique combining field ion microscopy and time-of-flight mass spectrometry for atomic-level analysis. It highlights the process of removing and analyzing individual atoms, the benefits of high spatial resolution, and its ability to map elemental and isotopic identities in volumes up to 100 nm. Additionally, it details the operational conditions, imaging systems, and advantages of field evaporation for achieving reliable chemical identification.