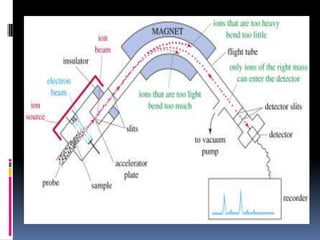
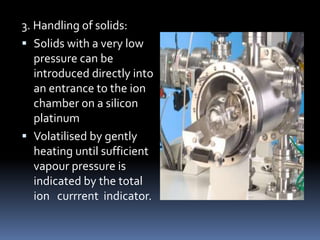
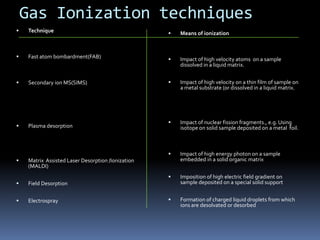
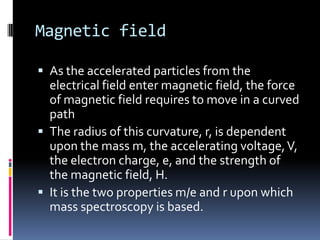
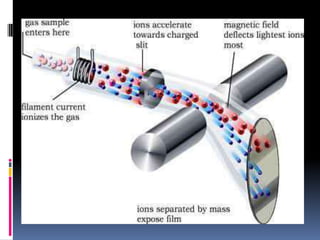
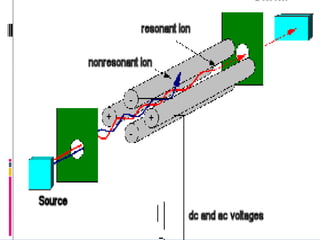
Mass spectrometry is an analytical technique that ionizes chemical species and sorts the ions based on their mass-to-charge ratio. It can provide qualitative and quantitative data about samples on an atomic or molecular level. The document discusses the principle, instrumentation, and applications of mass spectrometry. Key components of mass spectrometers include an ion source, accelerating system, magnetic field, ion separator, ion collector, and vacuum system. Mass spectrometry has applications in structure elucidation, detection of impurities, quantitative analysis, and various clinical and forensic analyses.