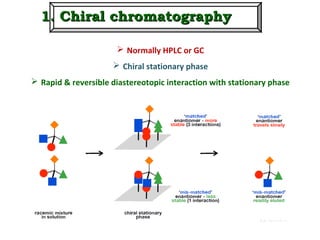
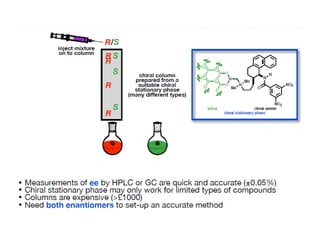
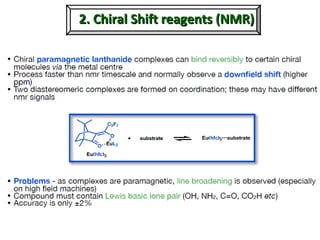
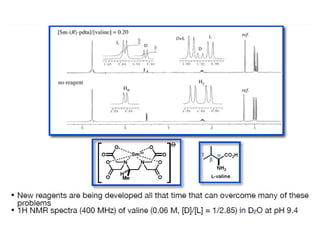
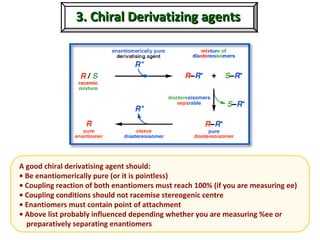
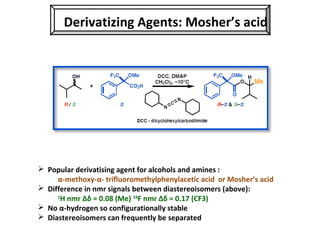
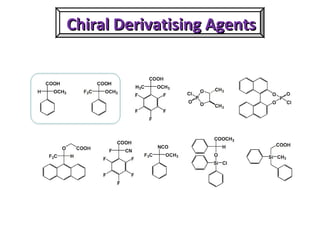
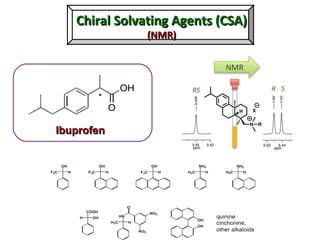
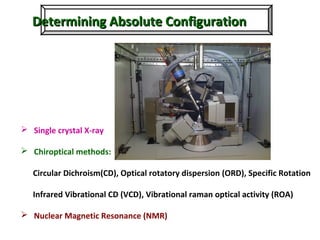
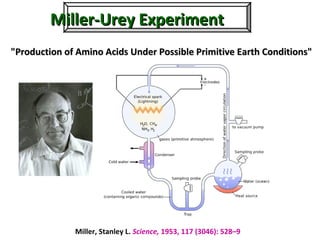
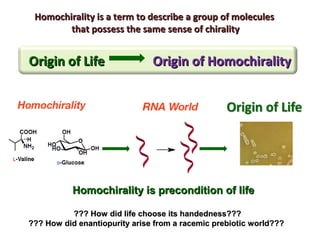
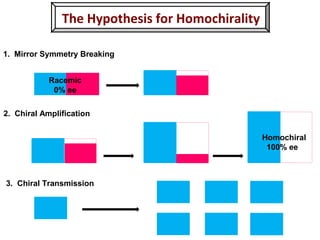
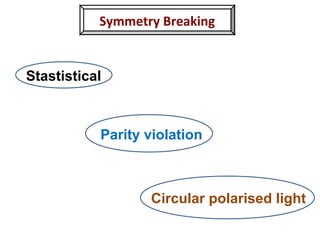
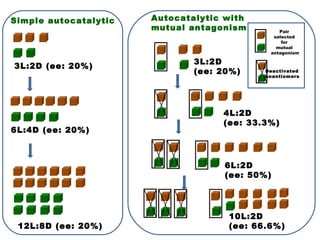
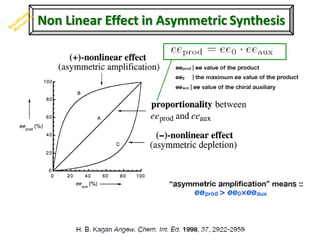
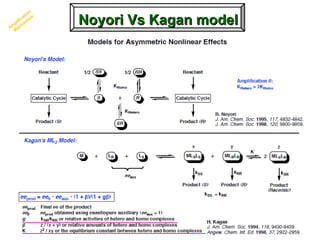
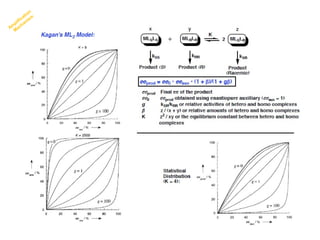
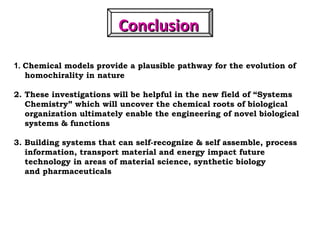
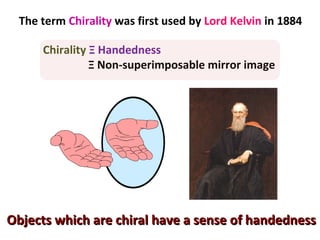
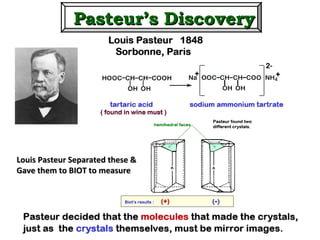
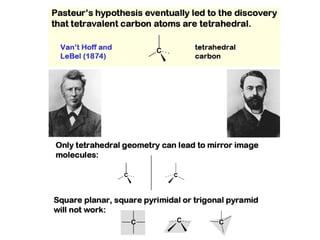
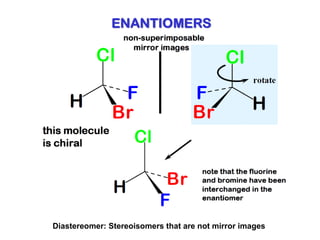
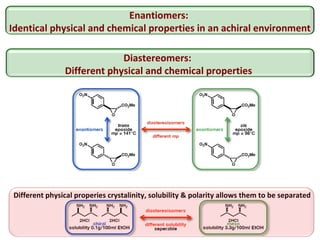
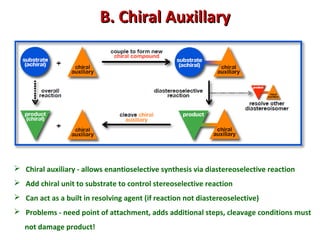
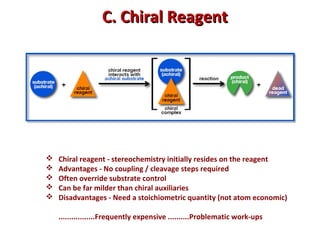
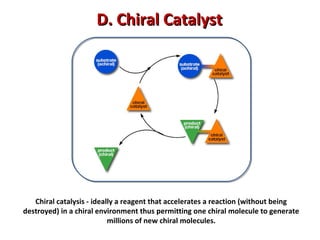
1. The document discusses chirality and its importance in the origin of life. It describes theories for how homochirality first arose, including symmetry breaking, autocatalytic amplification, and transmission of chirality through solid and solution phases.
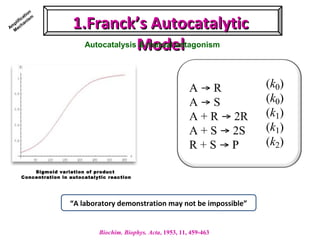
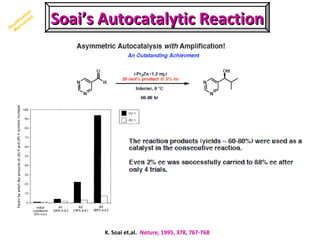
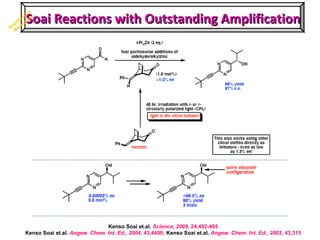
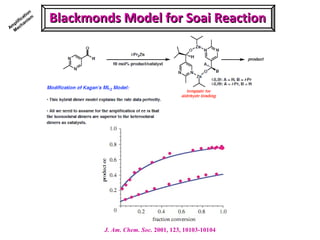
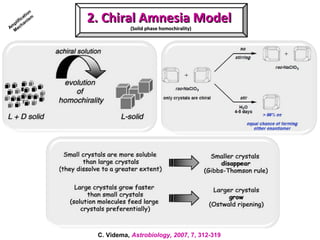
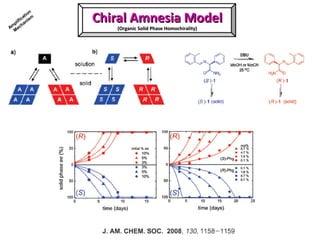
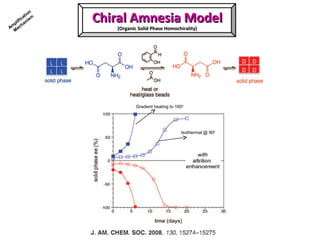
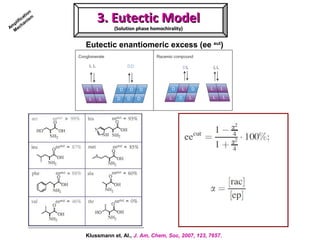
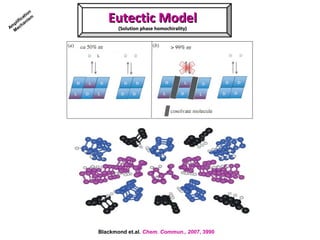
2. Specific models are examined, such as Frank's autocatalytic and Soai reaction models, chiral amnesia in organic solids, and eutectic solutions providing homochirality.
3. These chemical models provide plausible pathways for the evolution of homochirality in nature and will aid understanding the origins of biological organization and engineering novel biological systems.



![((Enantiomeric Excess (ee)Enantiomeric Excess (ee)))
Measuring ChiralityMeasuring Chirality
• Optical purity:Optical purity: a way of describing the composition of
a mixture of enantiomers
• Enantiomeric excess:Enantiomeric excess: the difference between the
percentage of two enantiomers in a mixture
optical purity is numerically equal to enantiomeric
excess, but is experimentally determined
x 100
[α]sample
Percent optical purity =
[α]pure enantiomer
x 100
[R] + [S]
[R] - [S]
Enantiomeric excess (ee) = = %R - %S](https://image.slidesharecdn.com/homochirality-170503085043/85/Homochirality-18-320.jpg)