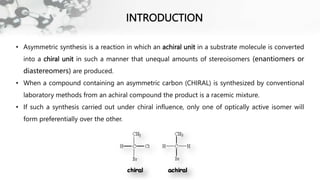
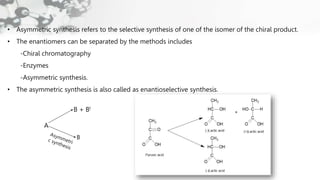
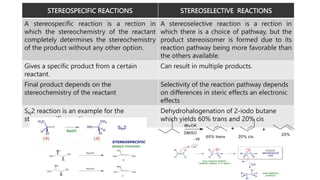
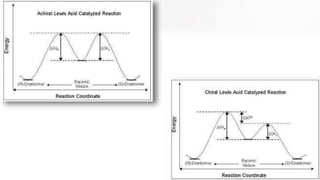
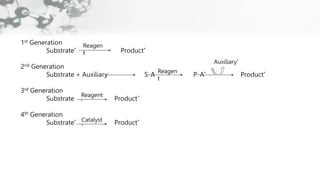
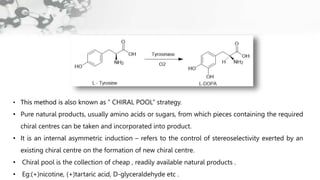
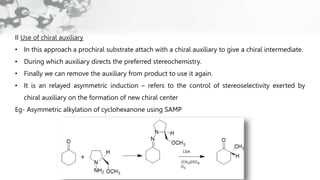
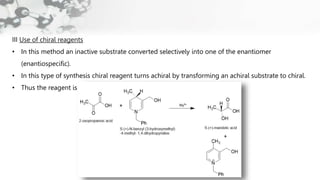
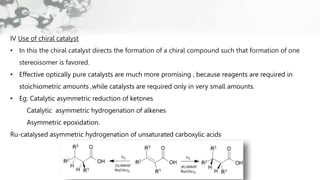
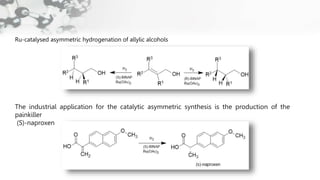
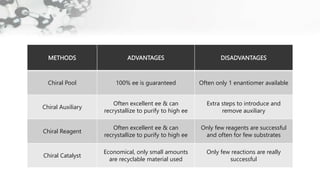
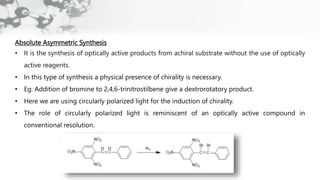
The document discusses methods of asymmetric synthesis, highlighting its importance in generating chiral products from achiral substrates through selective reactions. It covers stereoselectivity, mechanisms, four generations of asymmetric synthesis, and two main approaches: partial and absolute asymmetric synthesis. The text also elaborates on advantages and disadvantages of various methods such as using chiral pools, auxiliaries, reagents, and catalysts.