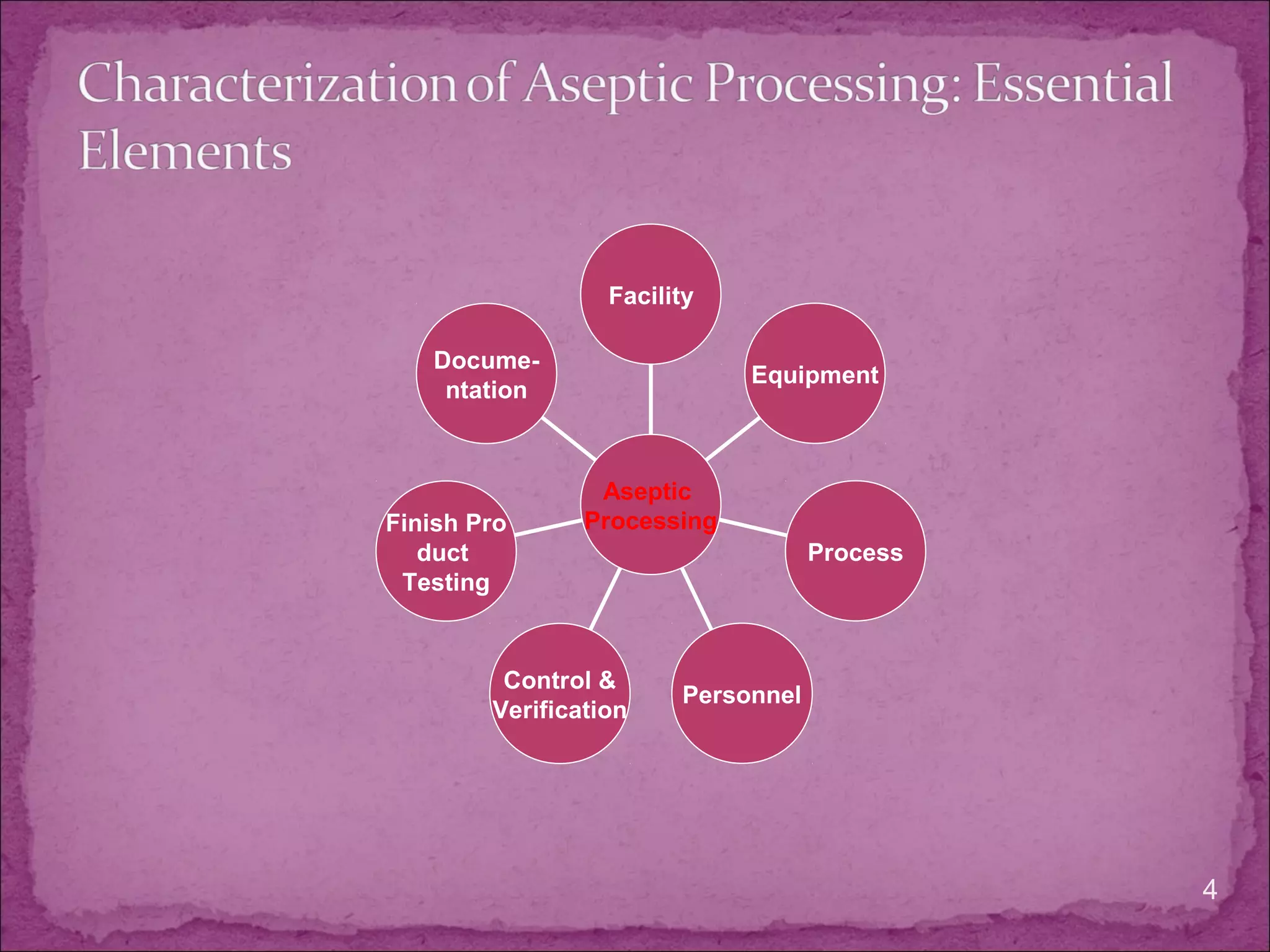
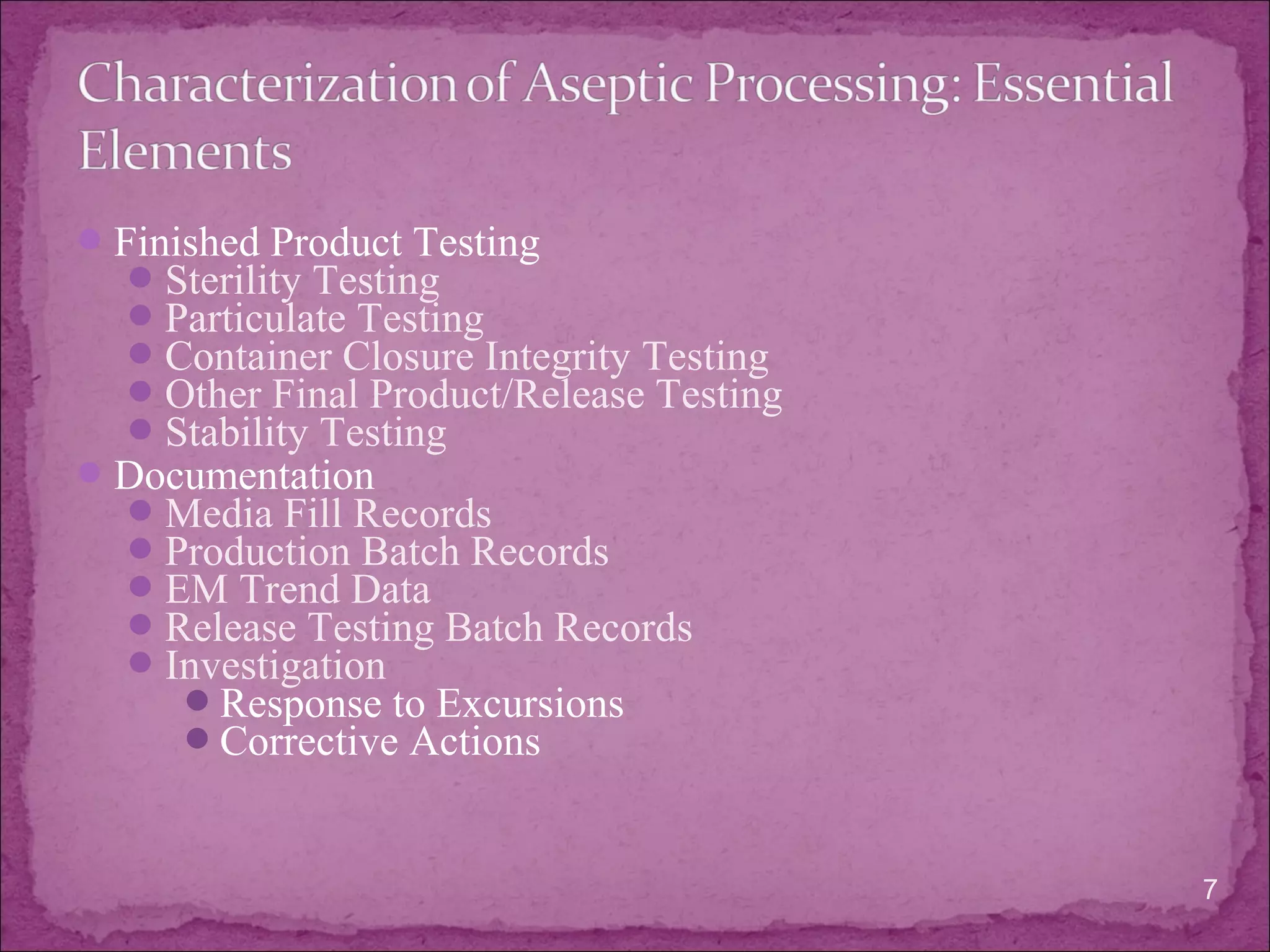
The document discusses aseptic processing operations. It describes the characterization of the aseptic process including microbial environmental monitoring, testing of water and air, and media and incubation conditions. The key aspects of the aseptic process are the facility design and control systems, equipment, personnel training, process validation, and finished product testing like sterility testing. Microbiological testing of water, air and media fills is important to ensure the sterility of pharmaceutical products manufactured through aseptic processing.