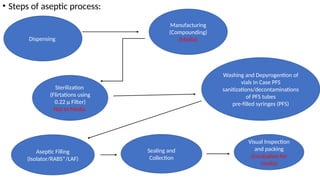
The document provides a detailed overview of media fill and process simulation in the context of manufacturing injectable products, emphasizing the significance of aseptic processing and regulatory requirements. It describes various types of injectable devices, the manufacturing and packing processes, and establishes the purpose of media fill testing, which is to validate the sterility of these processes. Additionally, it outlines specific regulatory guidelines and expectations for media fill testing to ensure contamination-free production and compliance with industry standards.