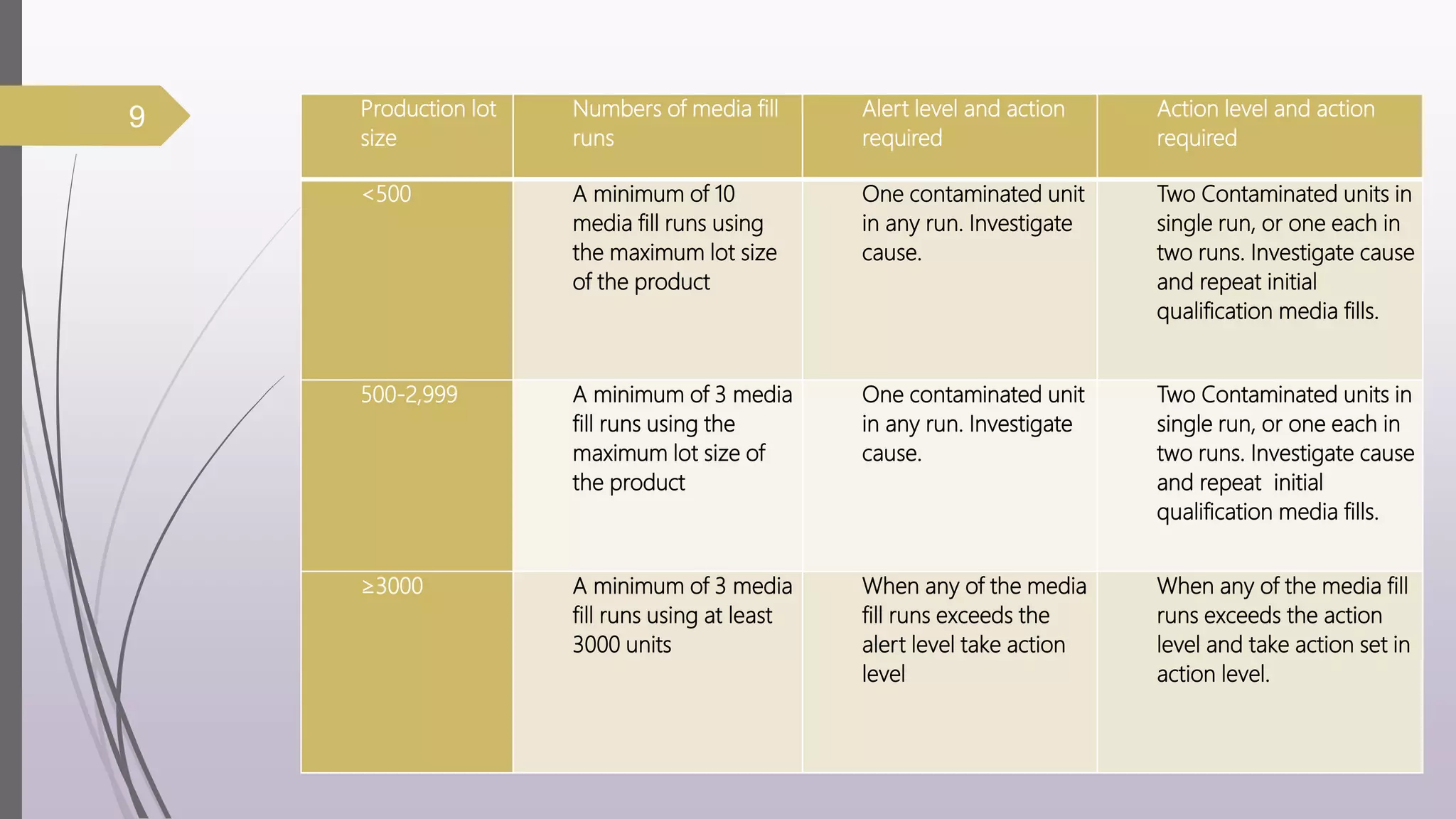
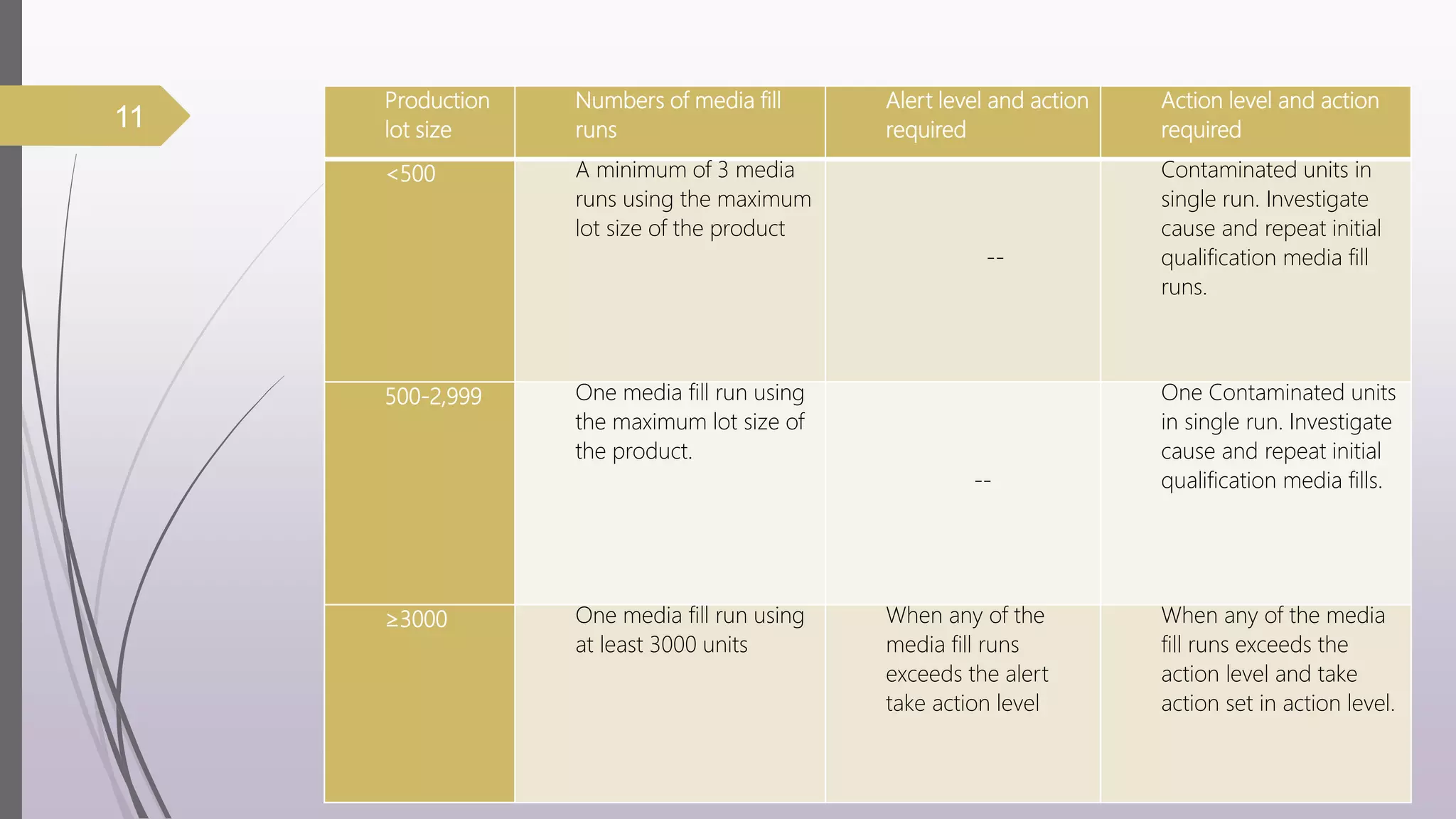
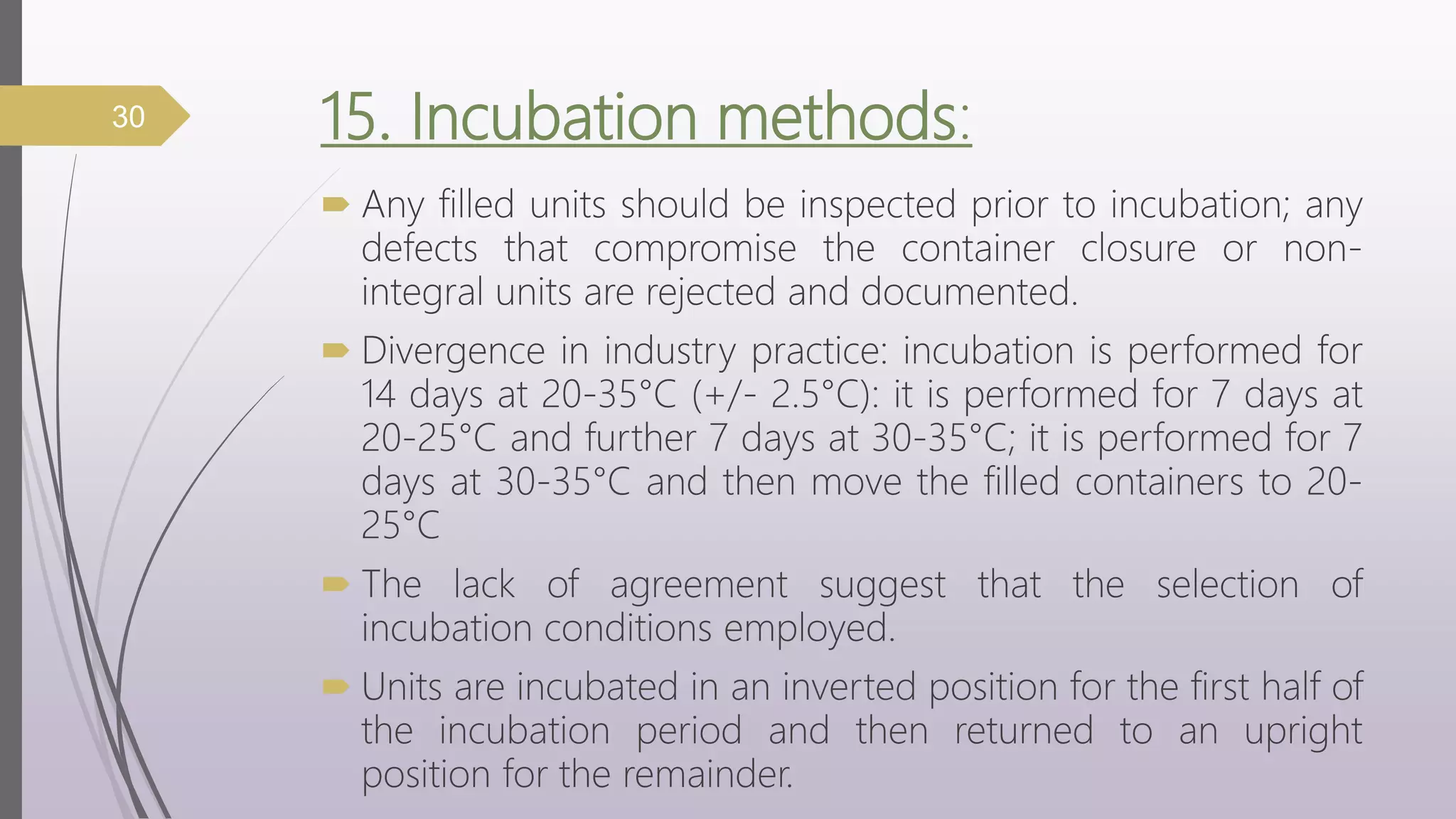
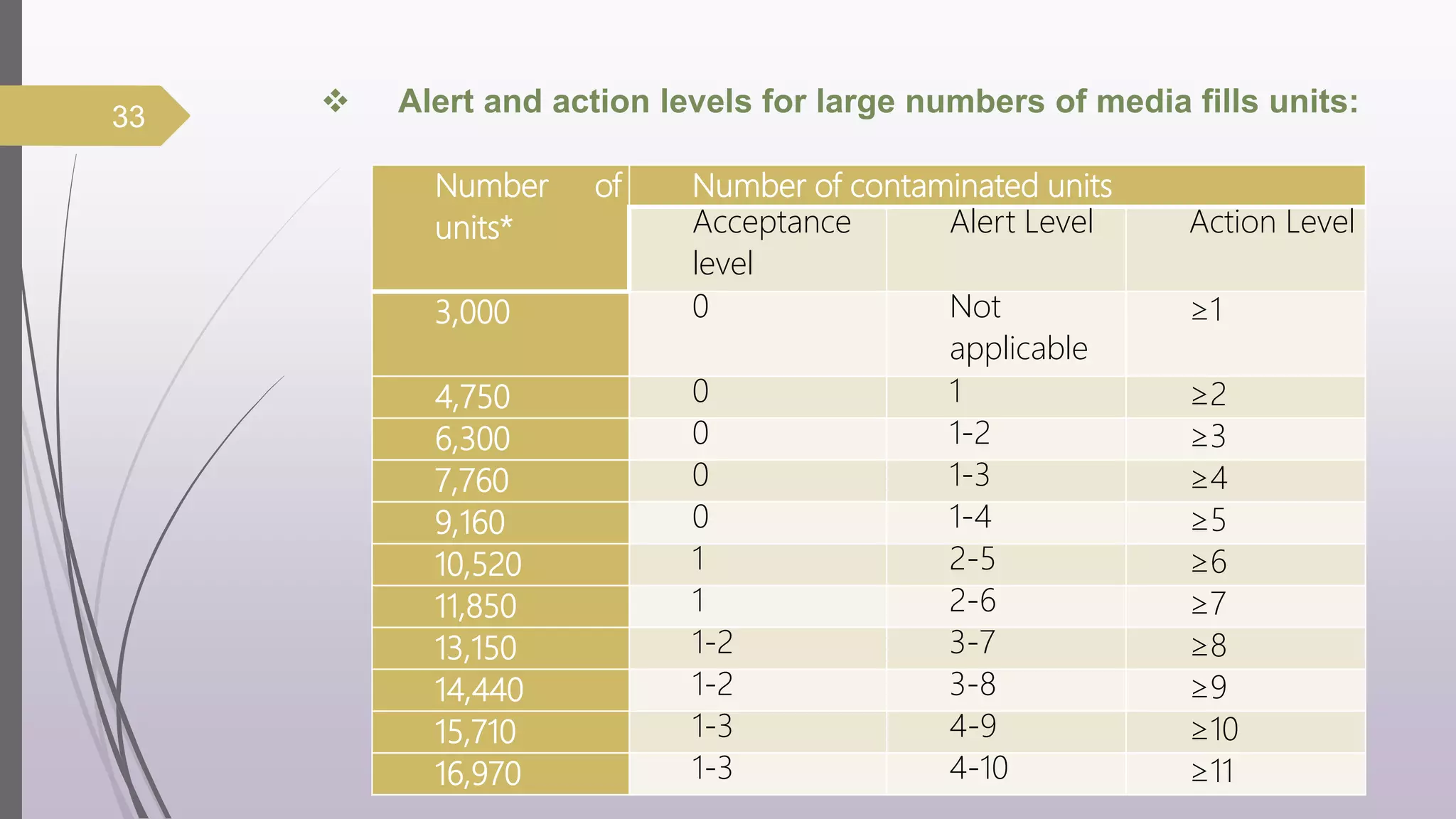
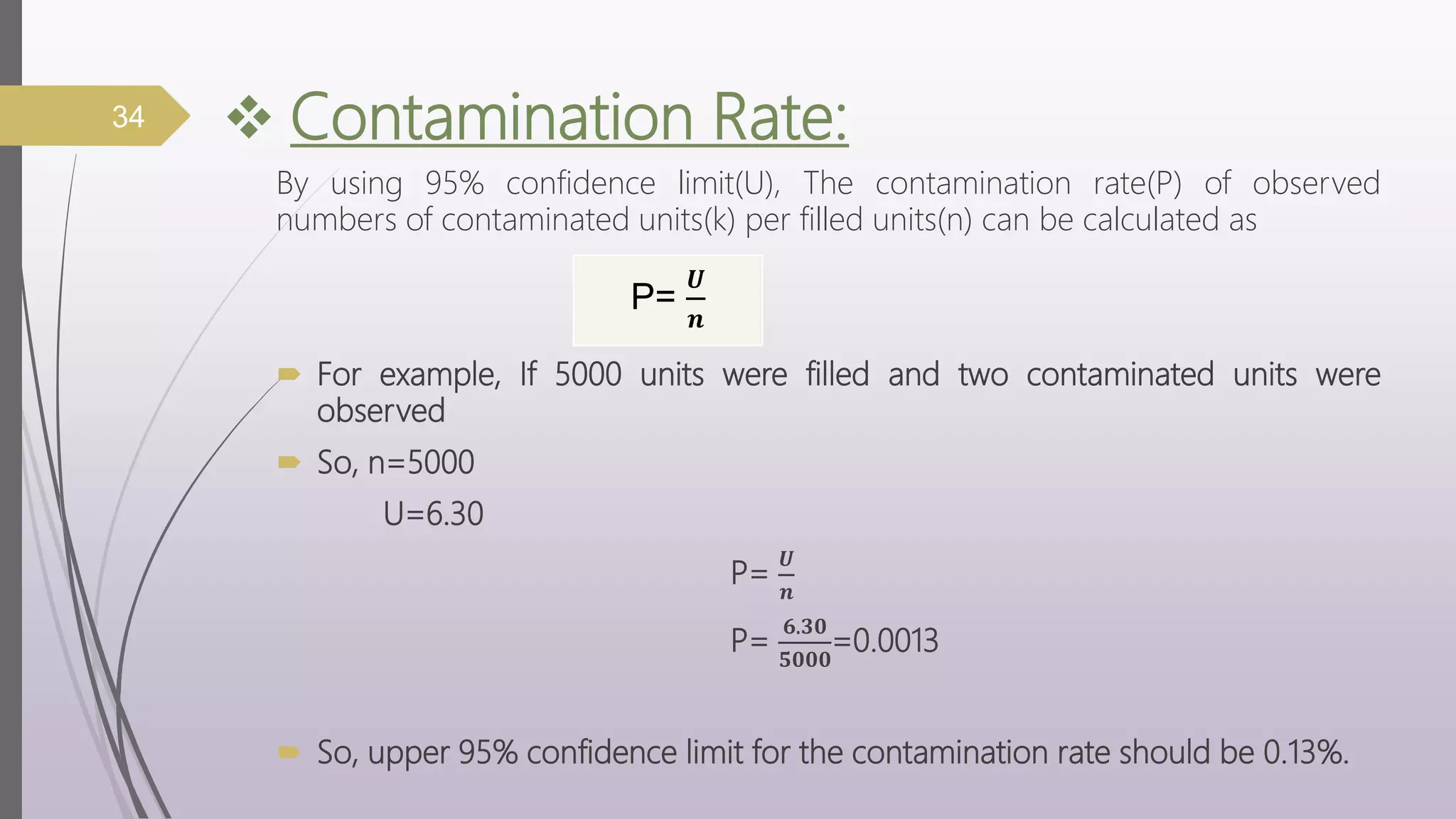
The document discusses media fill validation and USFDA guidelines on process validation. It provides details on conducting media fill tests, including study design, frequency of testing, media and container selection, filling procedures, incubation, and interpretation of results. Media fills are used to evaluate aseptic assembly, operator technique, and environmental controls. The document outlines various parameters that affect sterility, such as interventions, line speed, and incubation methods. FDA guidance recommends a lifecycle approach to process validation to continually monitor and improve manufacturing processes.