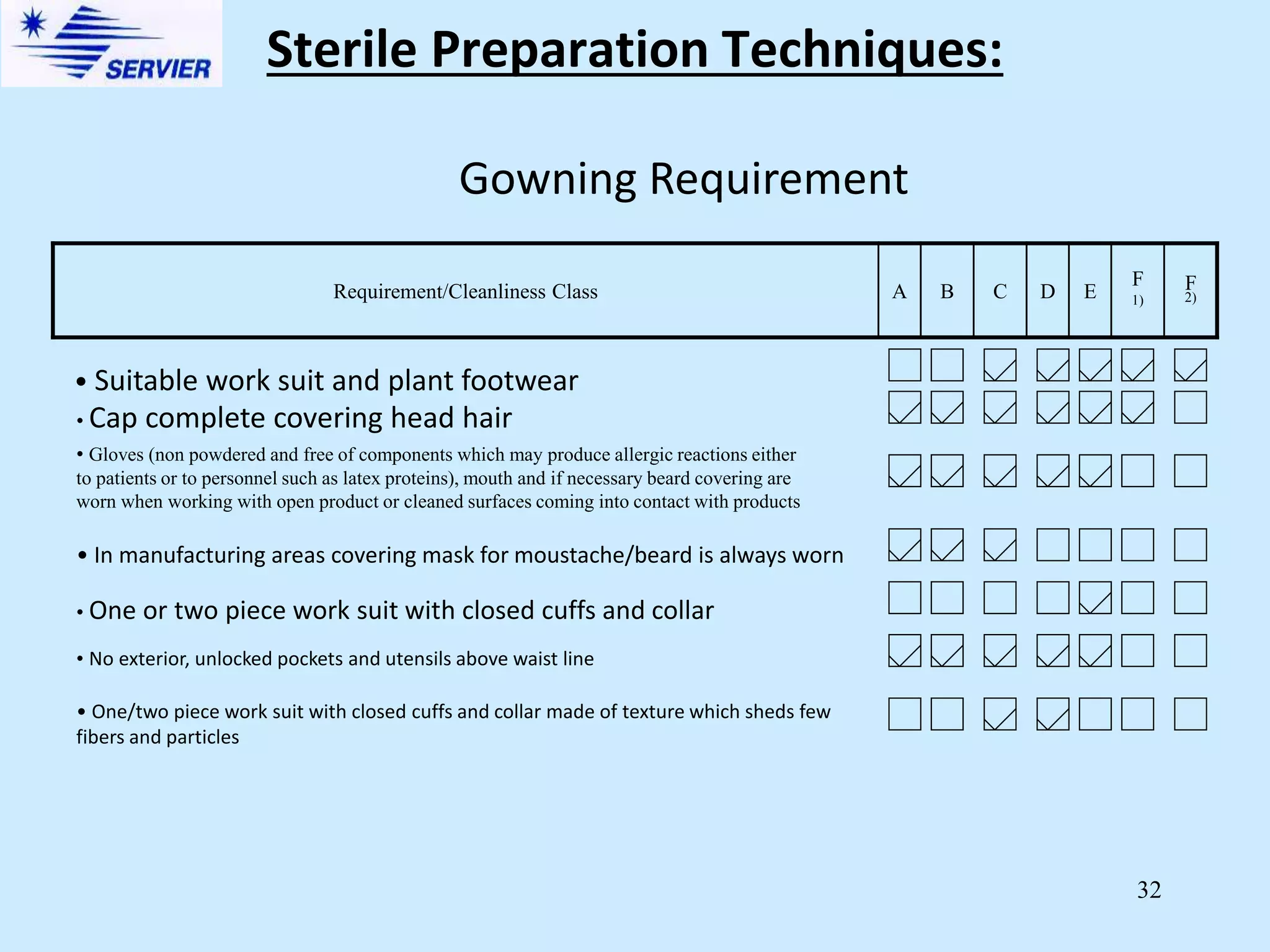
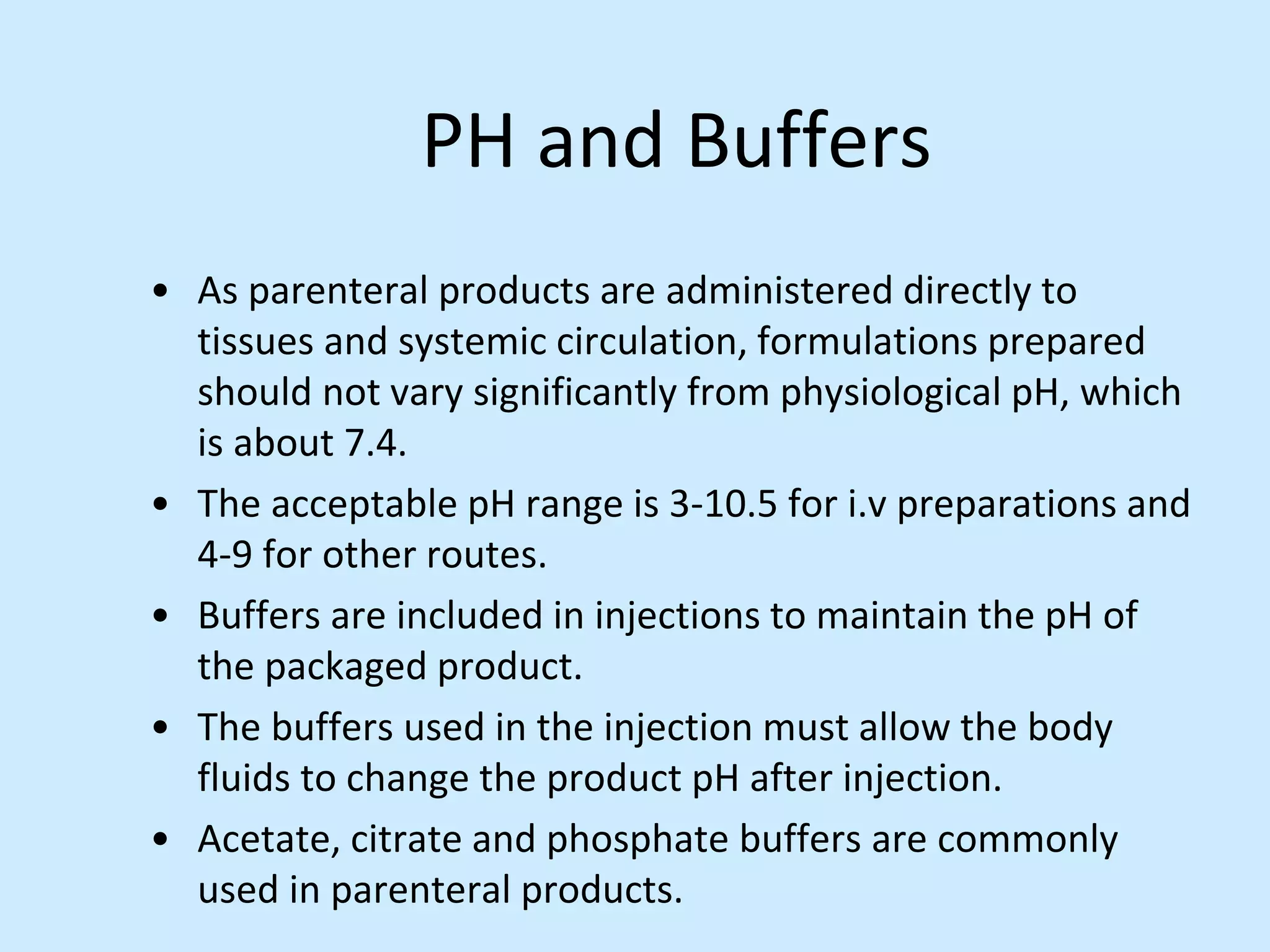
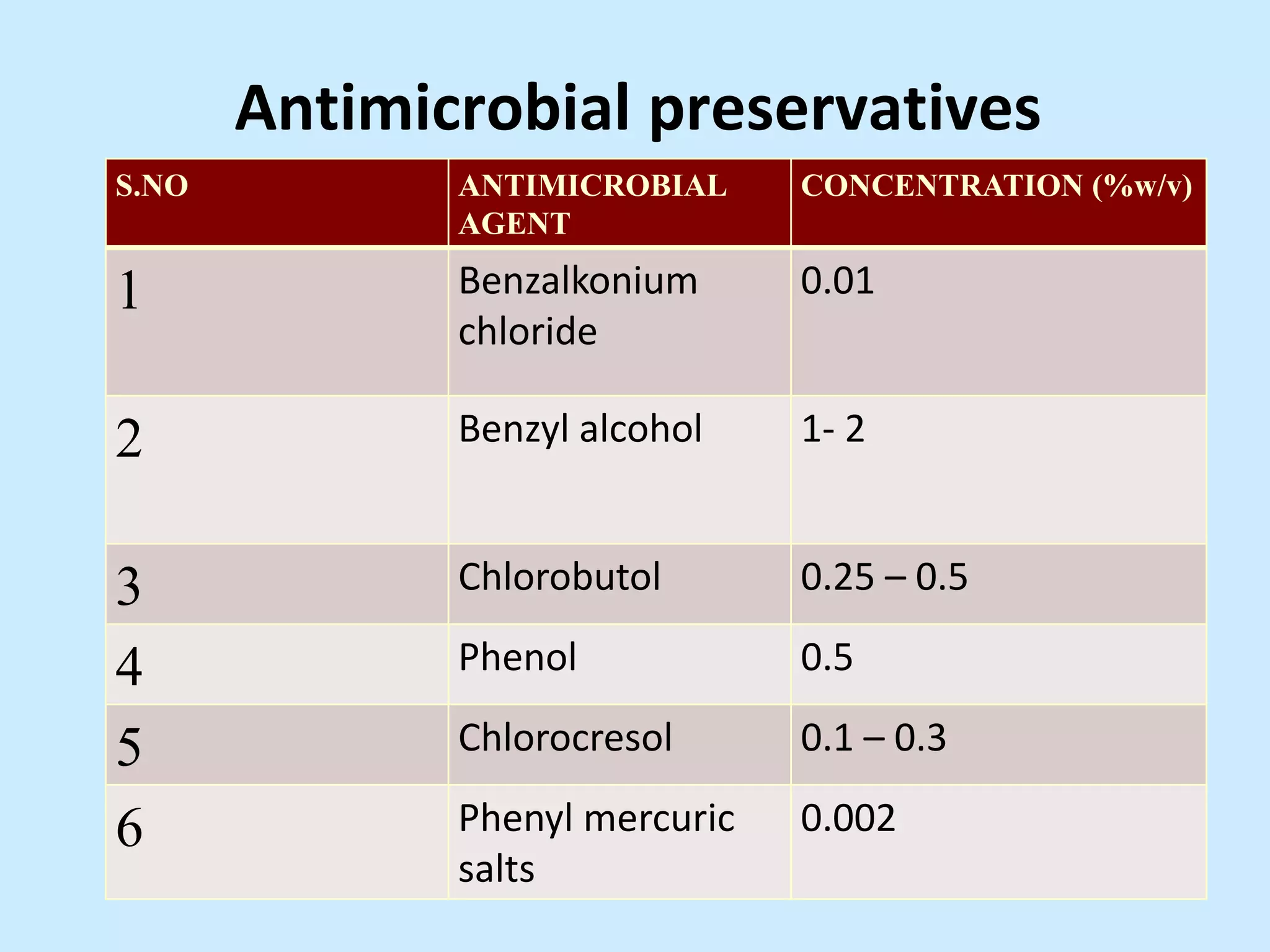
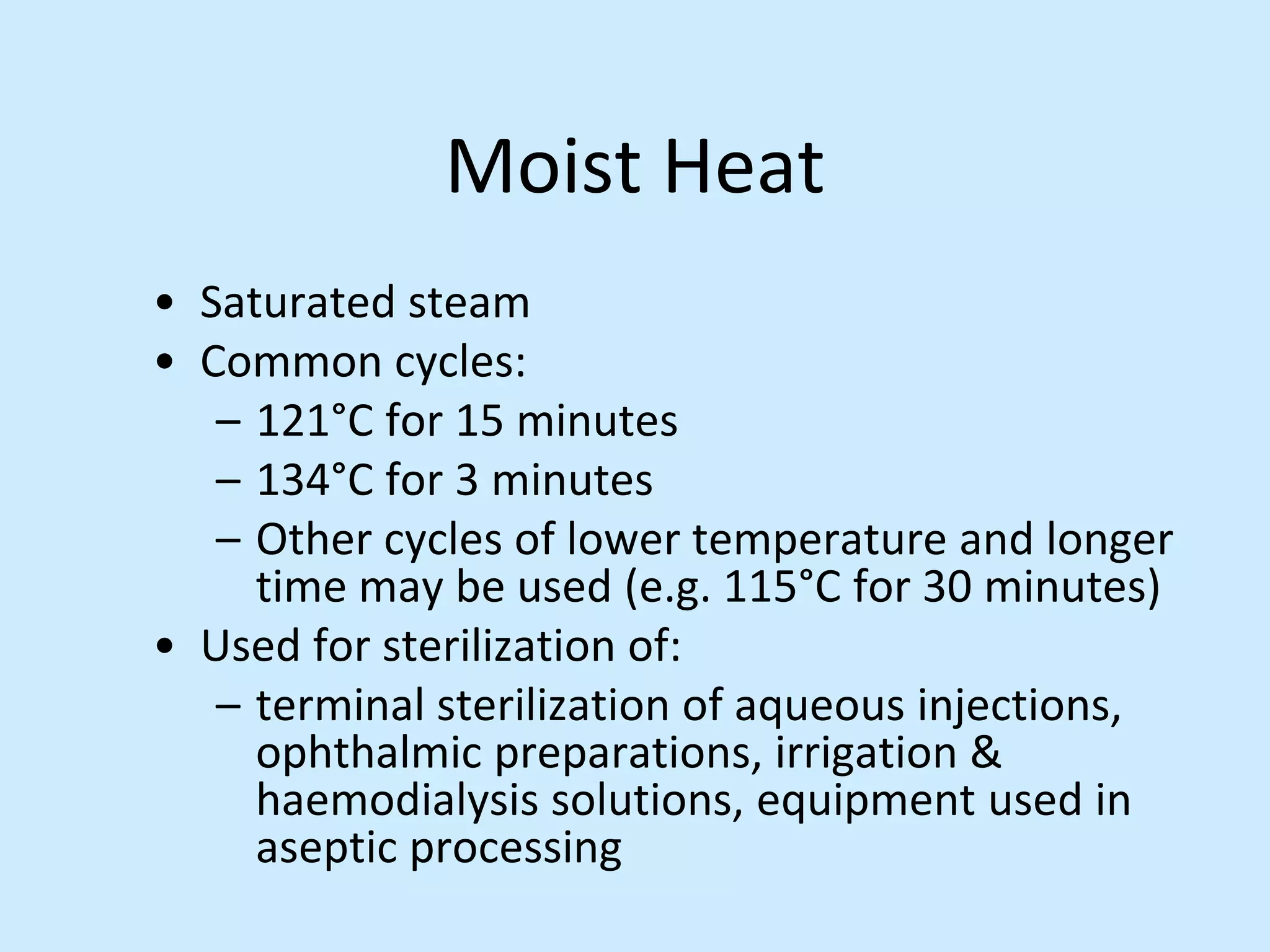
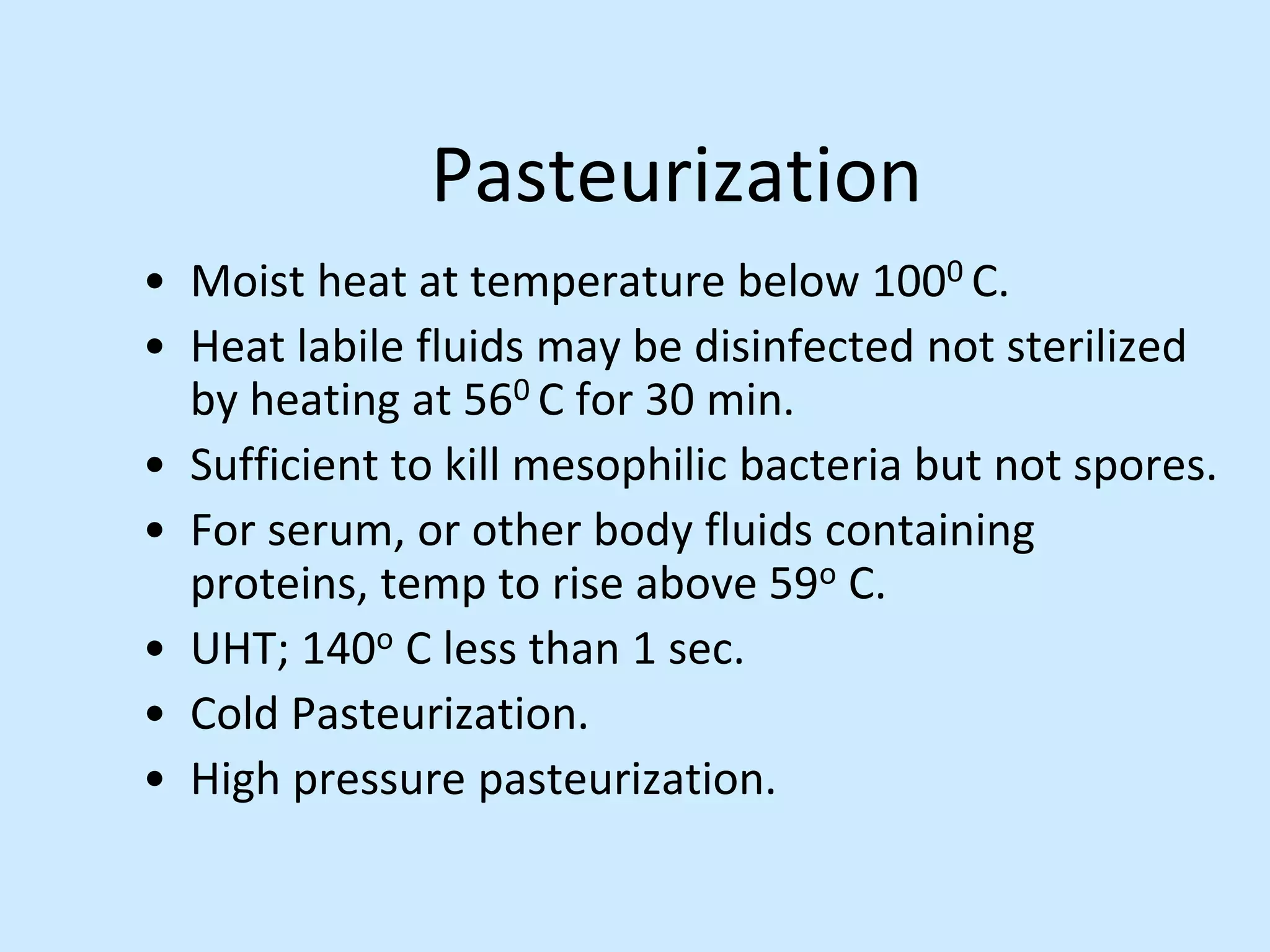
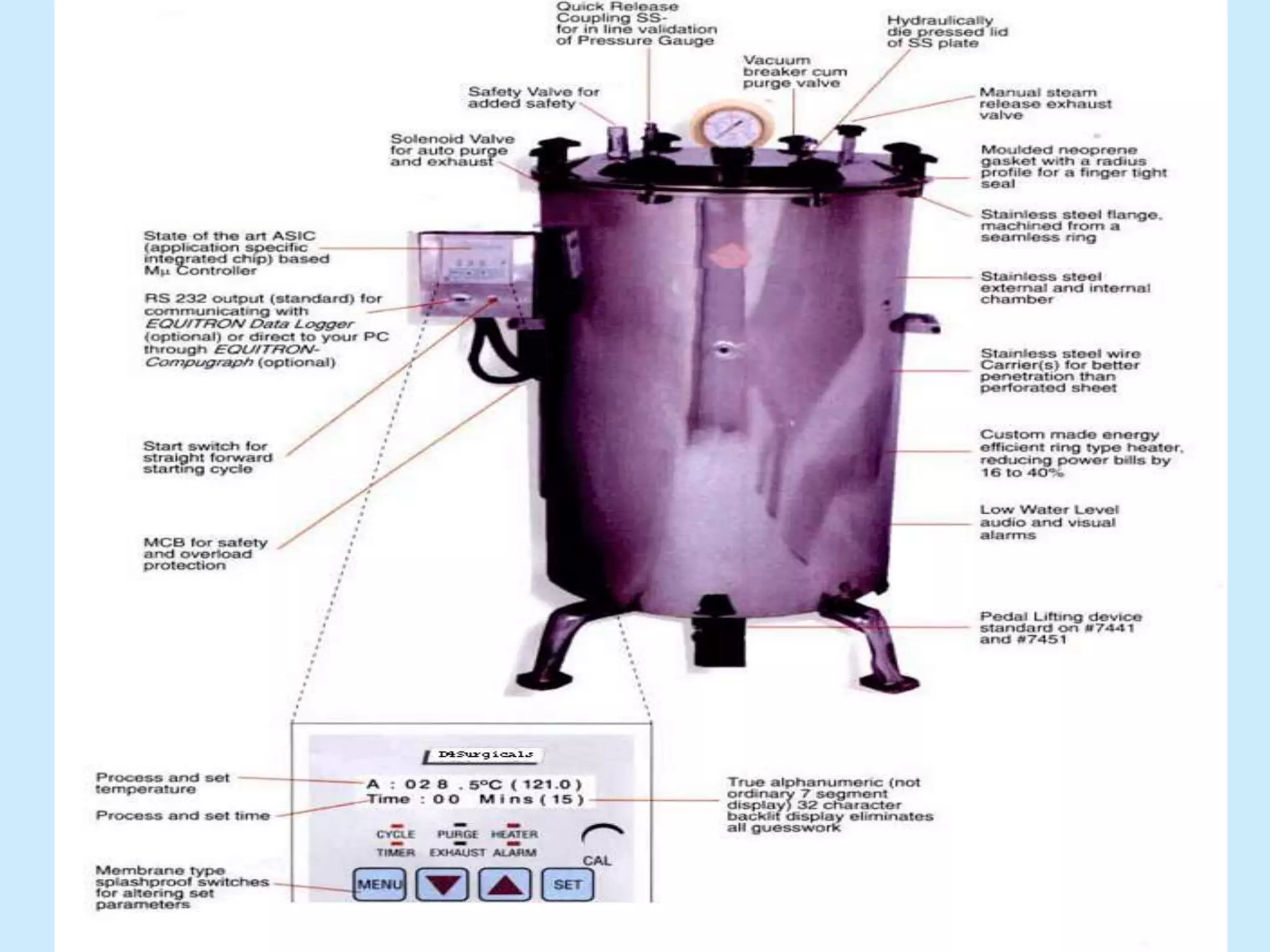
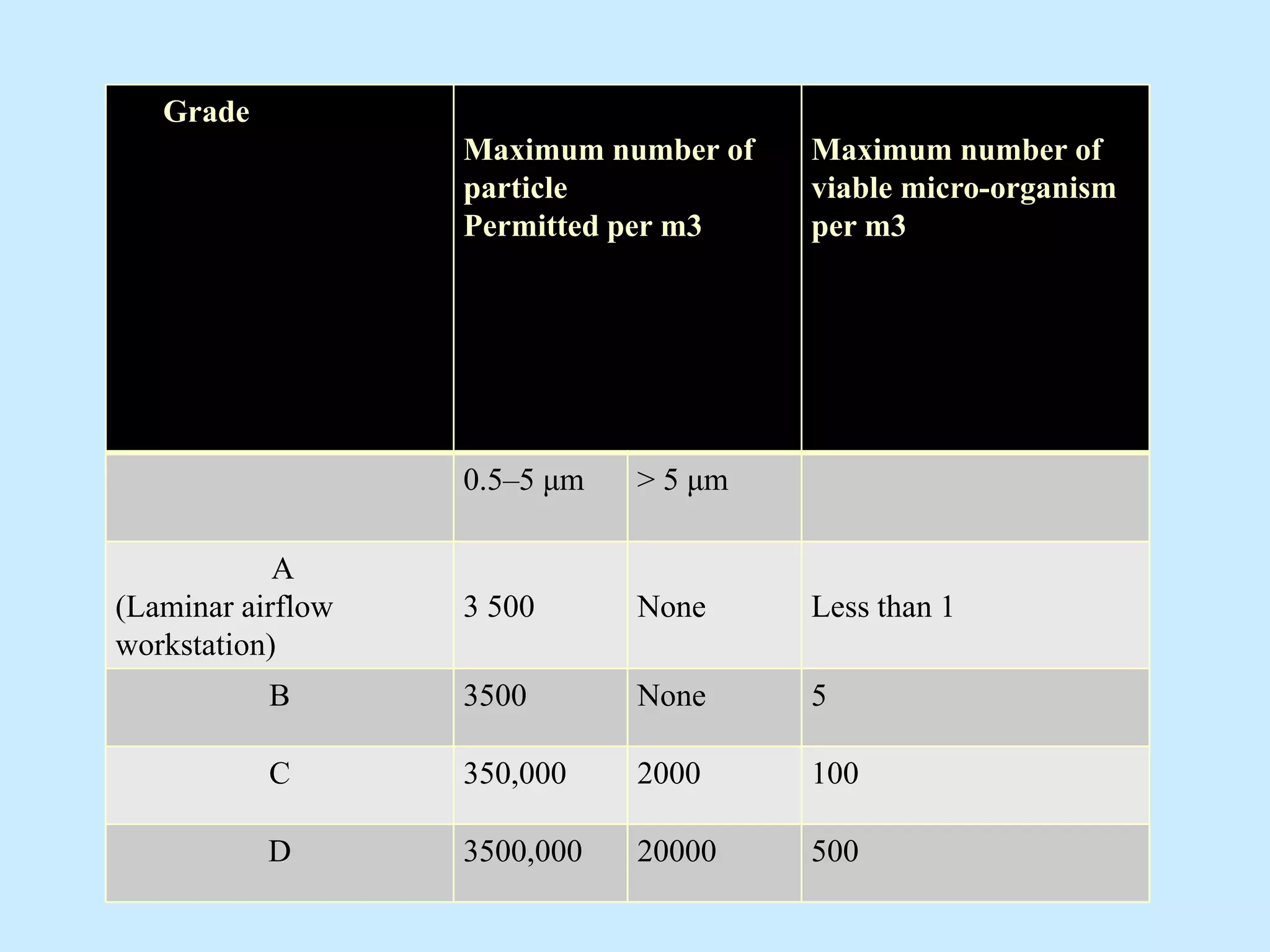
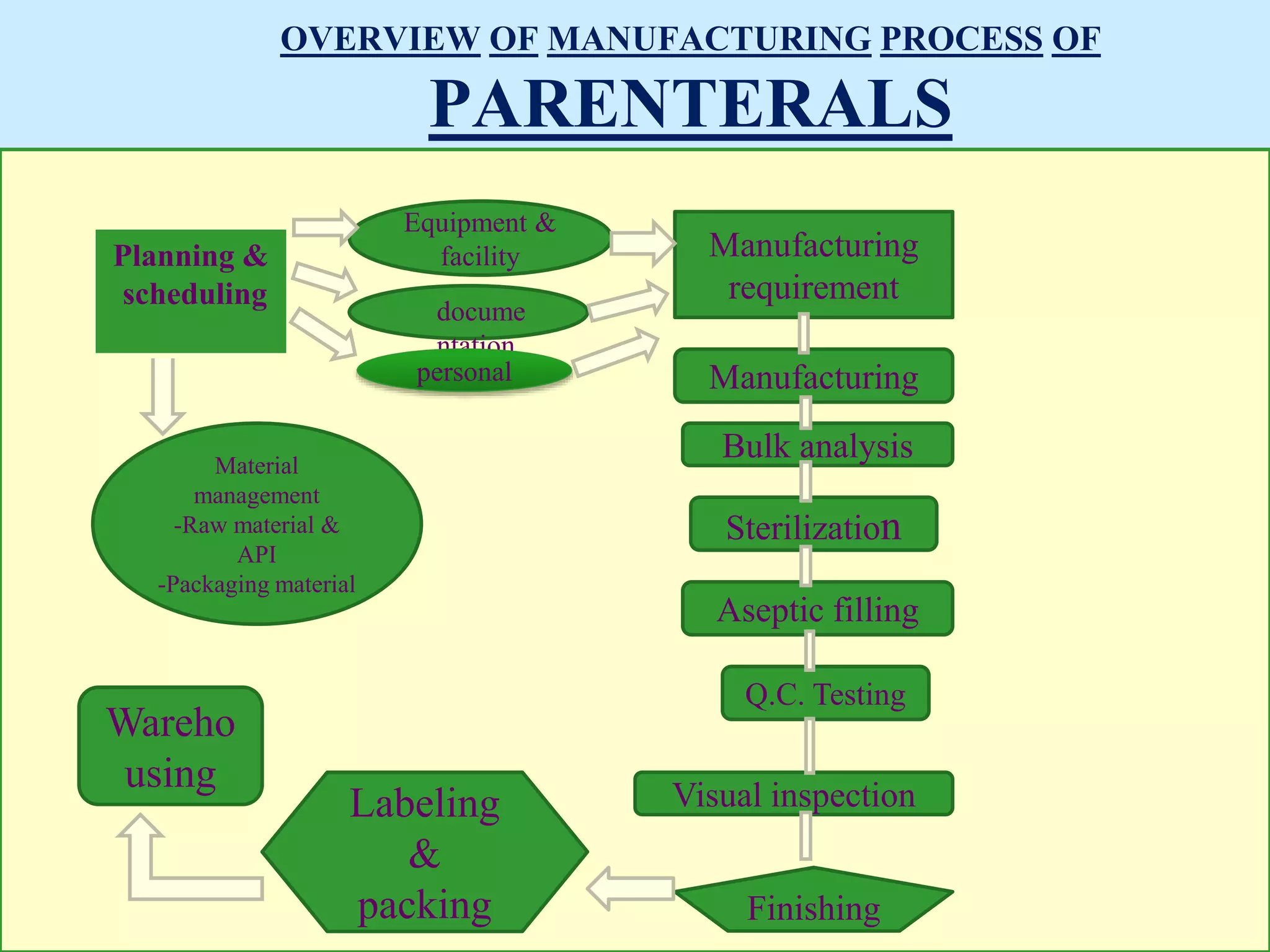
Sterile preparation techniques involve maintaining cleanliness levels from grades A through F to minimize contamination. Grade A areas have the highest level of protection for aseptic processing. Various sources of possible contamination like premises, air, personnel and materials are controlled. Personnel follow hygiene procedures and wear protective clothing appropriate for the cleanliness grade. Sampling locations are chosen based on factors like criticality and proximity to products to monitor environmental quality.