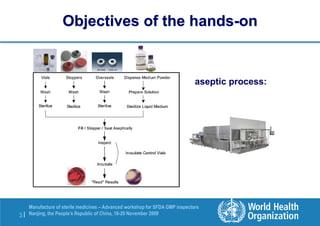
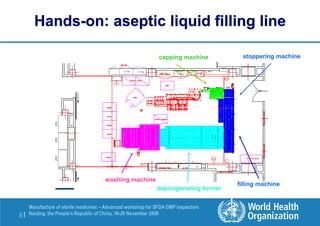
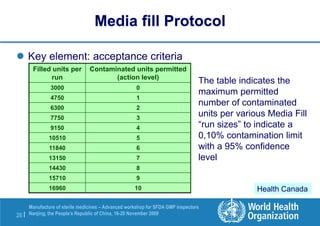
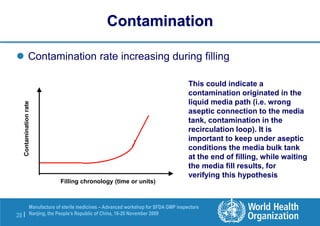
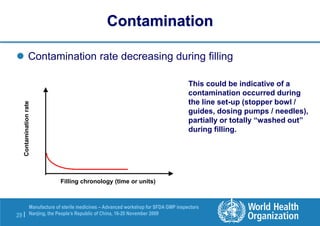
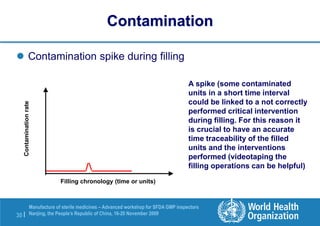
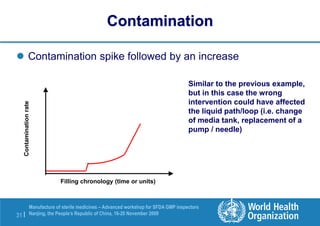
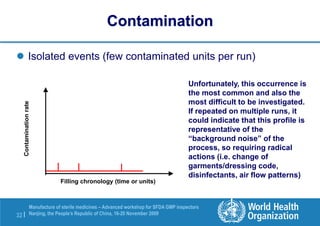
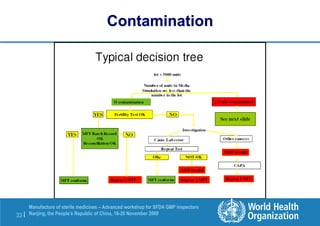
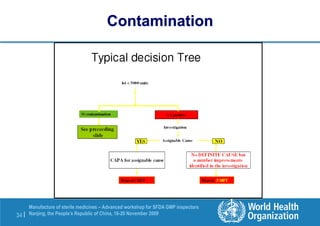
The document outlines the media fill protocol for aseptic process validation in the manufacture of sterile medicines, including key objectives such as evaluating aseptic process simulation and understanding regulatory expectations. It emphasizes critical elements like media culture, container size, and operator participation in media fill runs, highlighting the importance of stringent monitoring and evaluation protocols to minimize contamination risks. The document also discusses acceptance criteria for contamination rates and corrective actions to be taken in case of failures during the media fill process.