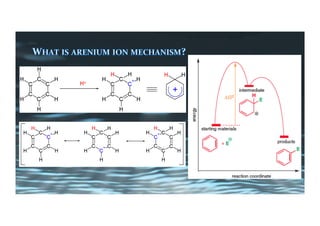
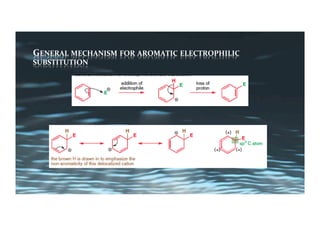
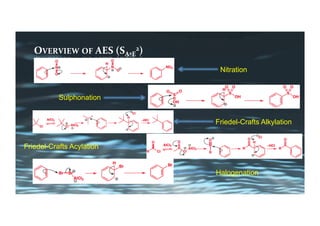
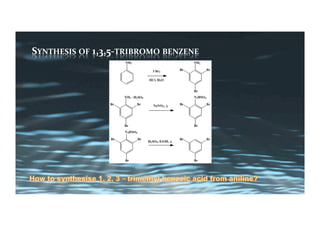
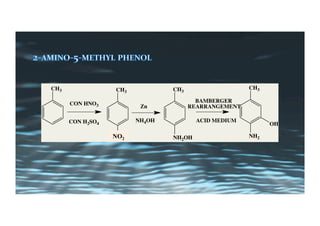
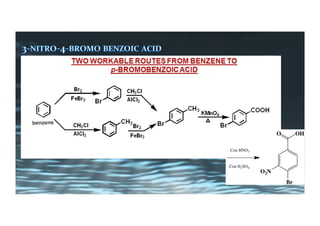
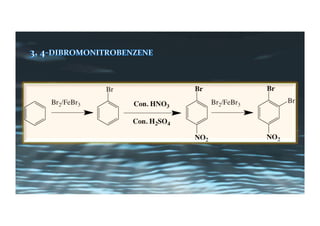
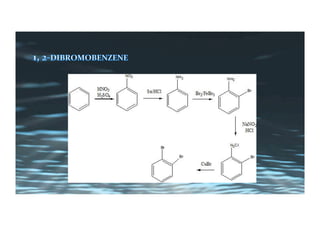
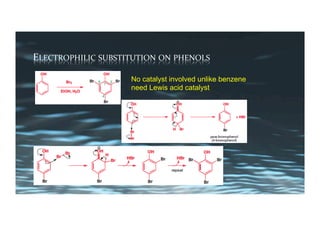
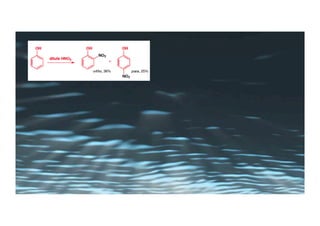
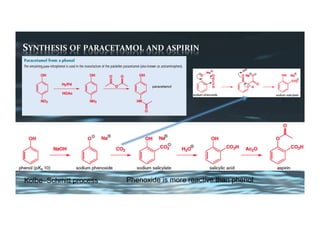
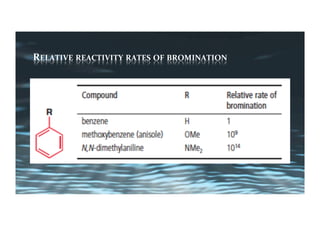
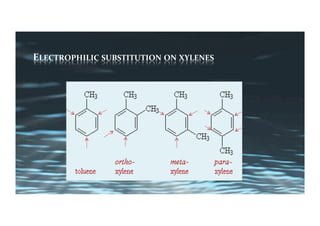
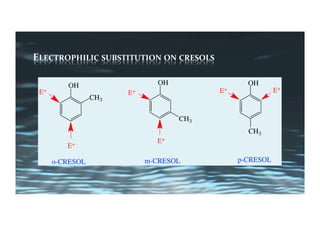
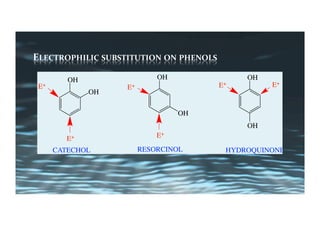
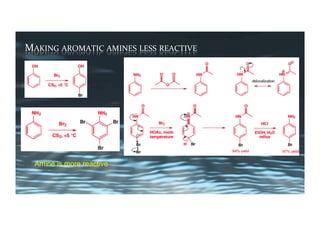
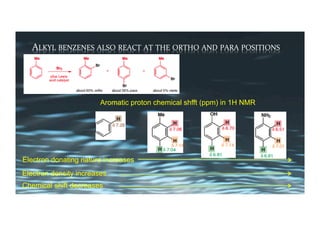
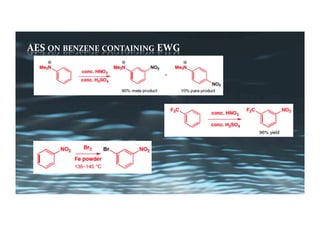
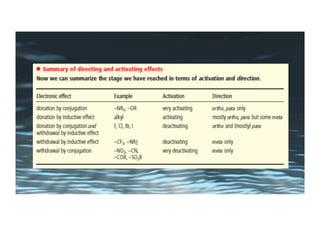
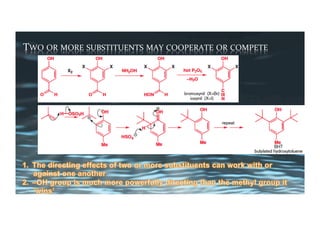
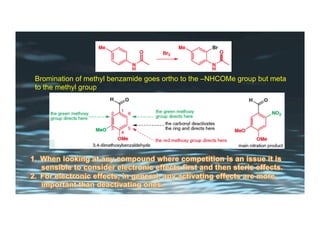
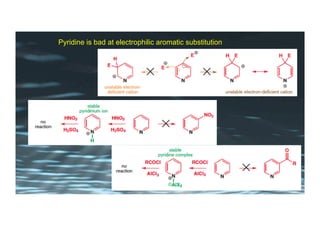
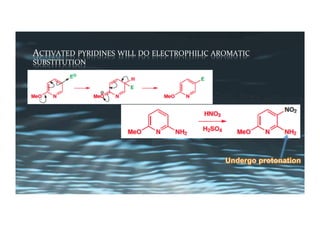
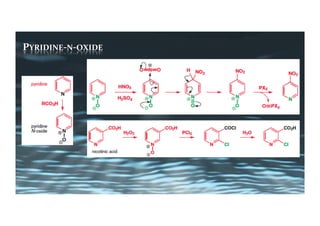
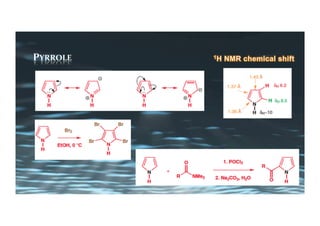
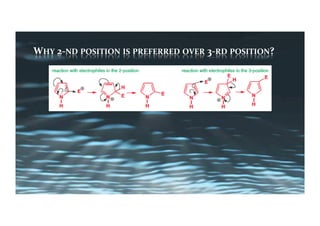
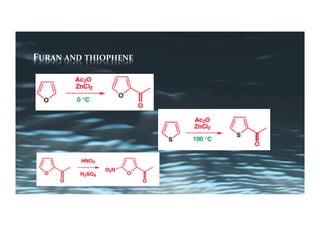
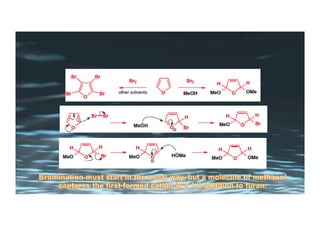
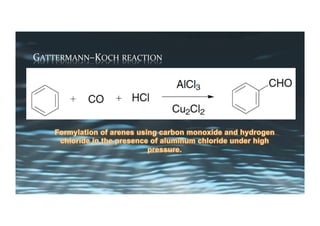
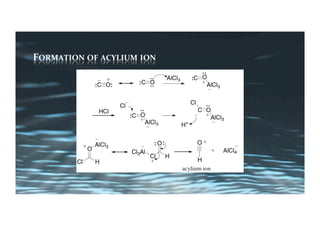
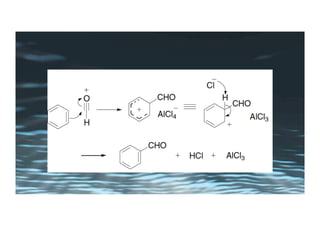
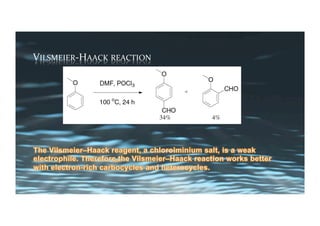
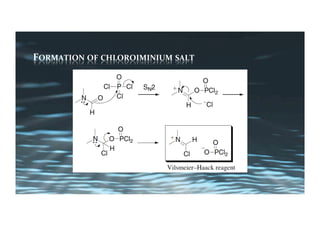
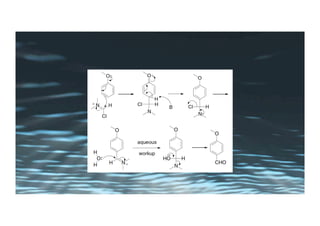
This document provides an overview of aromatic electrophilic substitution reactions (AES). It defines important terms like arenium ions, electrophiles, nucleophiles and discusses the effects of substituents on reactivity. The mechanisms of common AES reactions like nitration, sulfonation, Friedel-Crafts alkylation and acylation are covered. The document also discusses topics like the mesomeric and inductive effects of substituents, the synthesis of tribromobenzene, and the relative reactivities of benzene and substituted benzenes in bromination. Examples of AES on phenols, xylenes, cresols and other aromatic compounds are provided.