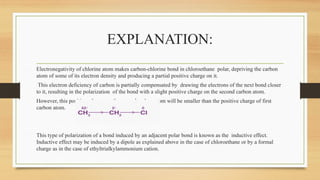
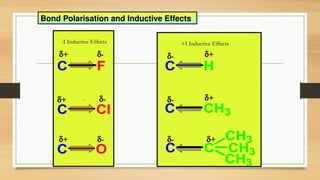
The inductive effect is the electron-withdrawing or electron-donating effect transmitted through sigma (σ) bonds in a molecule due to differences in electronegativity between atoms.
---
🔹 Definition:
The inductive effect is the permanent shifting of electrons in a sigma bond caused by the electronegativity difference of atoms, resulting in partial charges within the molecule.


















![8:Effect of substituent on the acid strength of aliphatic acids:
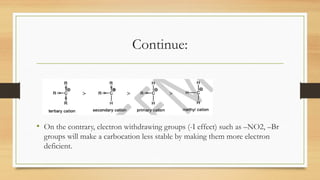
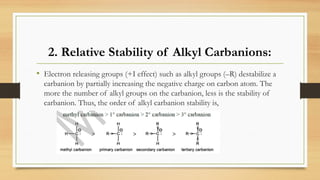
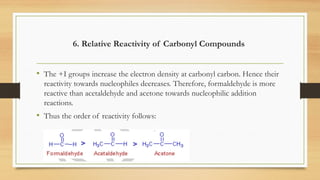
• [1]. Acidic strength decreases as +I effect of the alkyl group increases and vice versa.
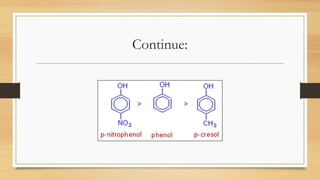
• Acid strength: HCOOH > CH3COOH > (CH3)2CHCOOH
• [2]. Acidic strength decreases as the number of halogen atoms decreases and vice versa.
• Acid strength: Cl3CCOOH > Cl2CHCOOH> ClCH2COOH > CH3COOH
• [3]. Acidic strength decreases as the distance of the halogen from carboxylic group increases and vice
versa.
• Acid strength: CH3CHClCOOH > CH2ClCH2COOH
• [4]. Benzoic acid is stronger than acetic acid due to –I effect of phenyl group.](https://image.slidesharecdn.com/inductiveeffect-250616101658-2e1b9421/85/INDUCTIVE-EFFECT-slide-for-first-prof-pharamacy-students-26-320.jpg)