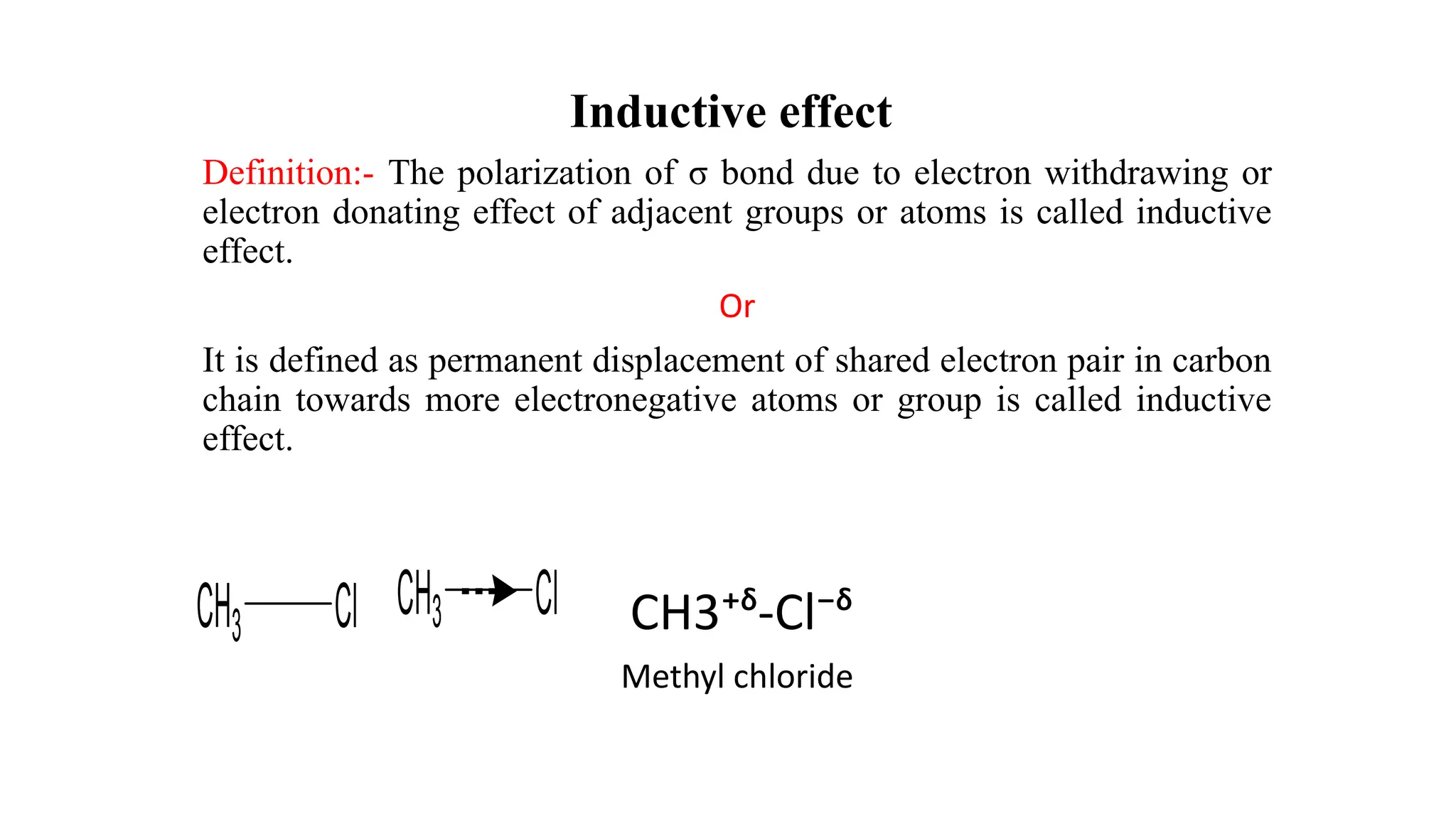
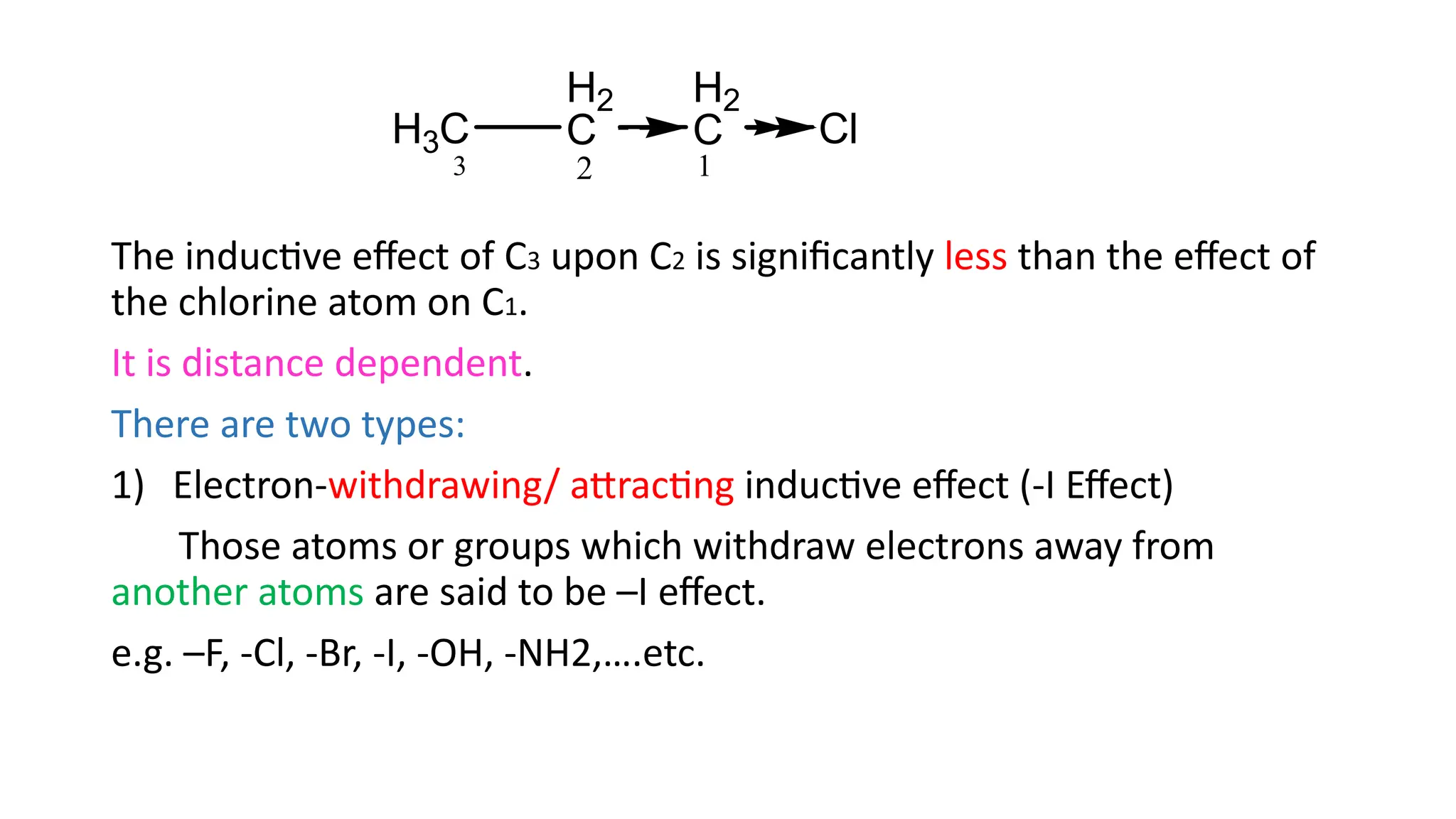
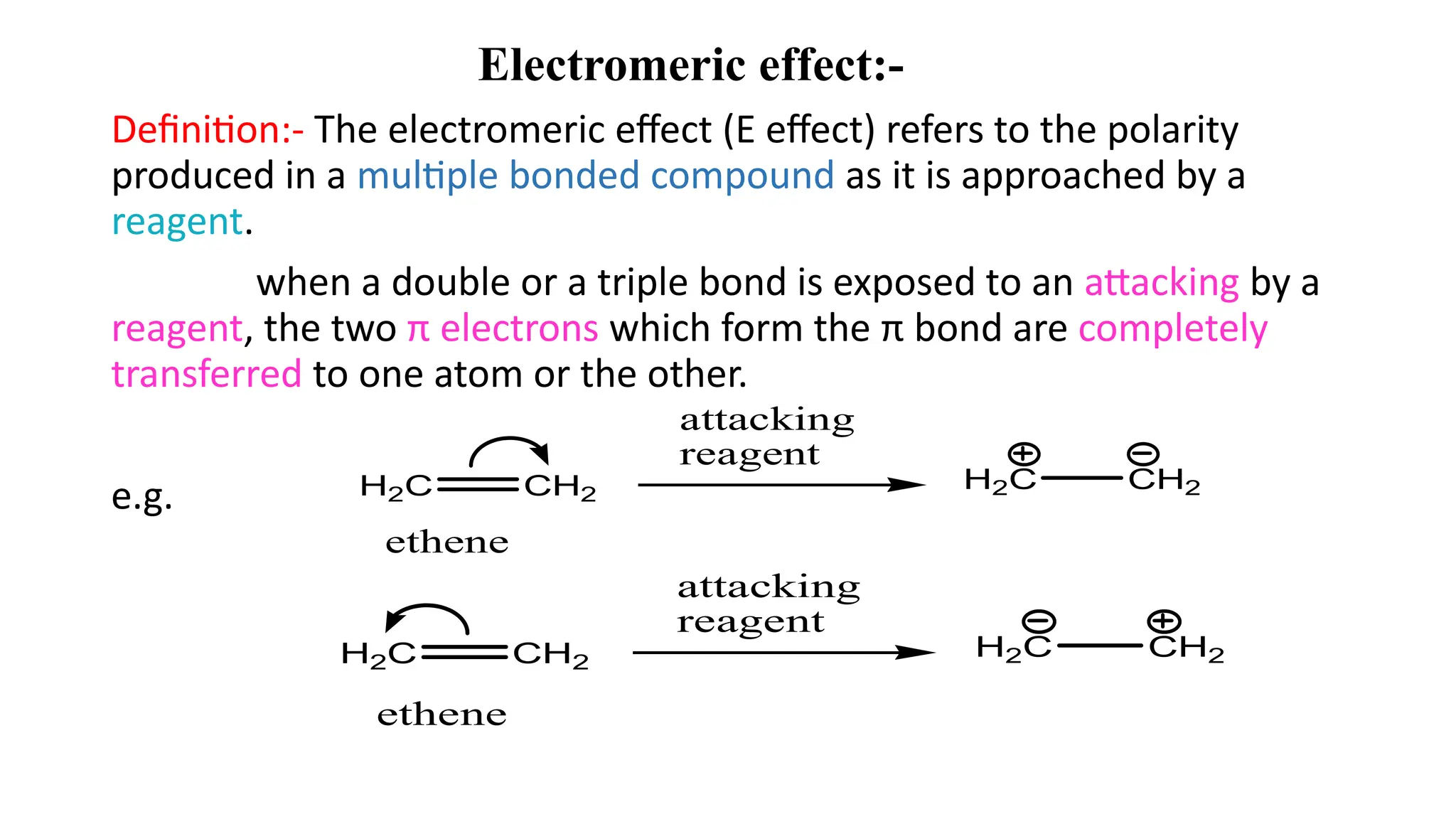
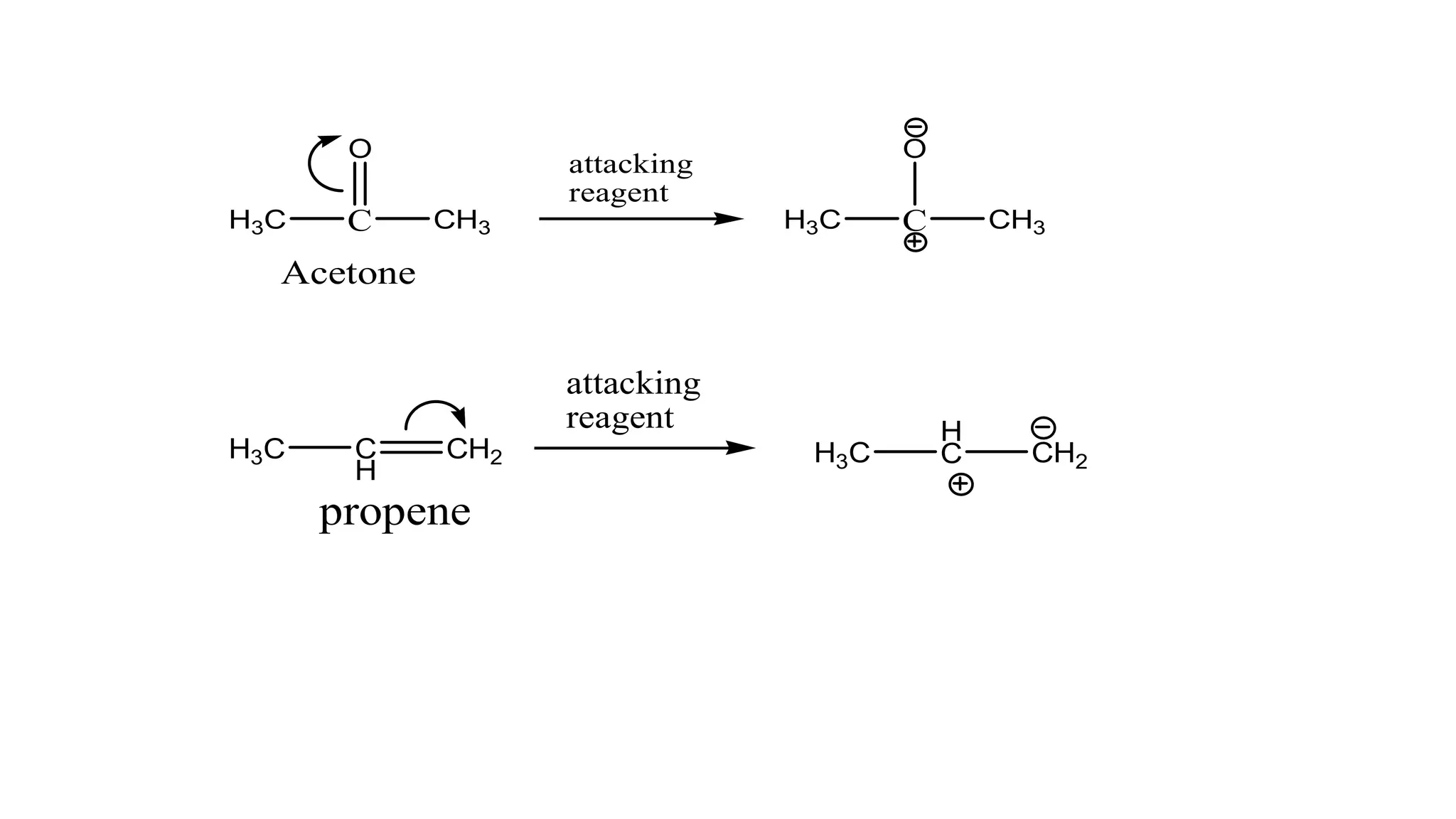
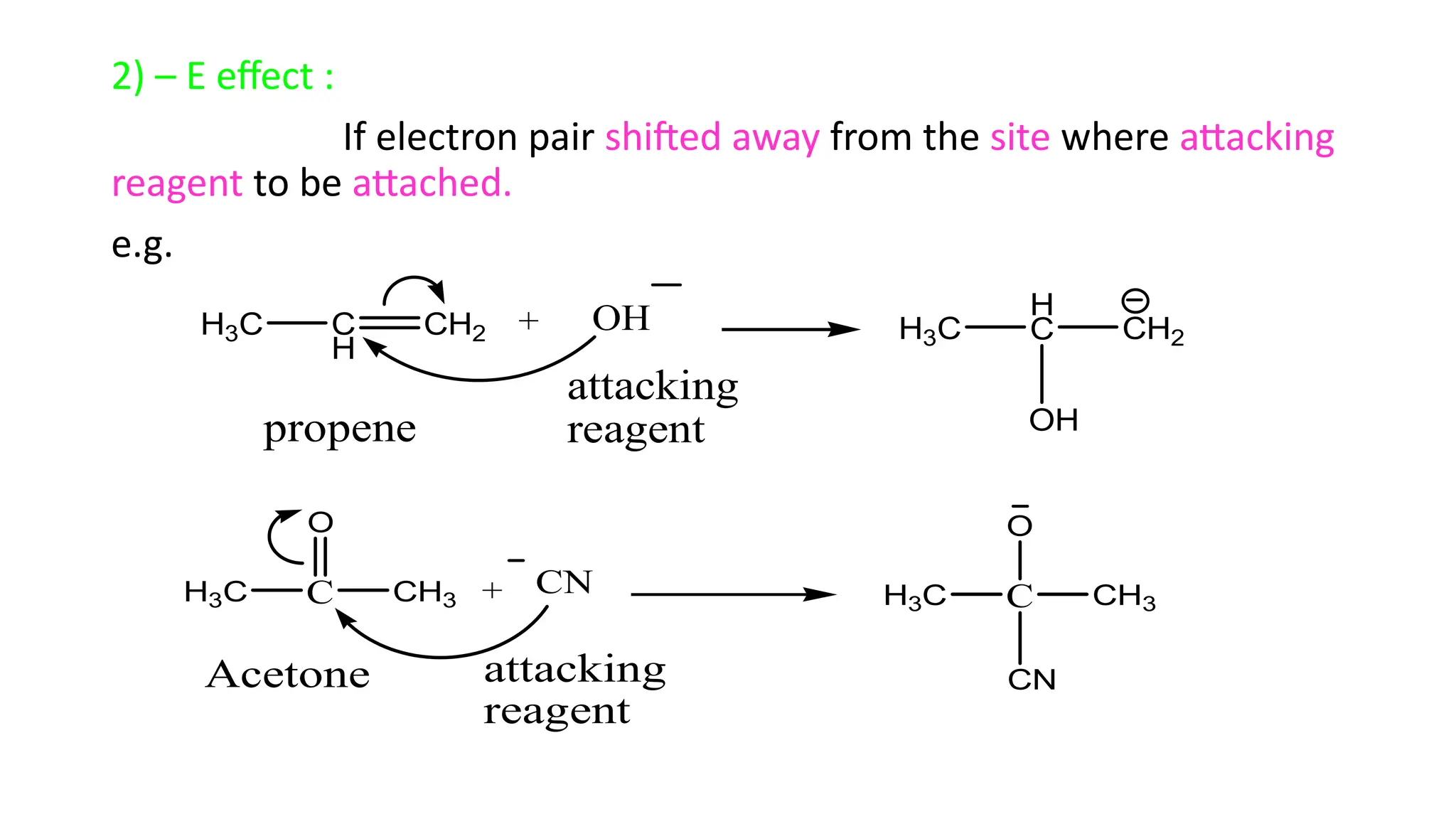
The document explains the inductive effect, which is the polarization of σ bonds due to the electron-withdrawing or donating effects of adjacent atoms/groups, featuring types such as -i and +i effects. It also covers the electromeric effect, related to the polarity produced in multiple bonds when a reagent approaches, detailing the positive (+e) and negative (-e) types. Both effects demonstrate electron displacement in chemical bonds, with the inductive effect being permanent and the electromeric effect being temporary and only occurring in the presence of a reagent.