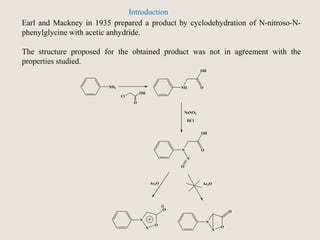
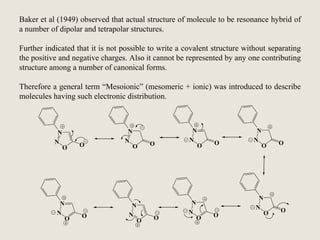
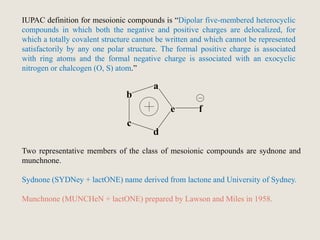
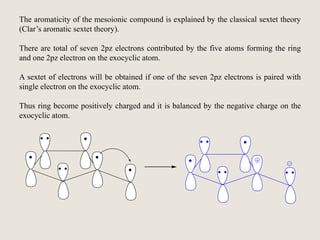
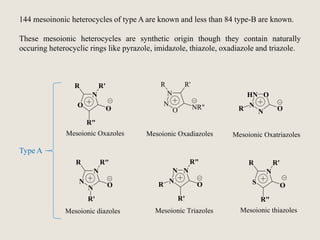
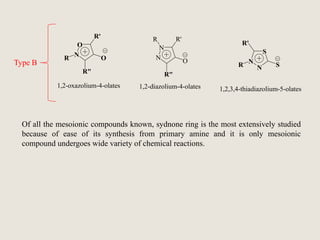
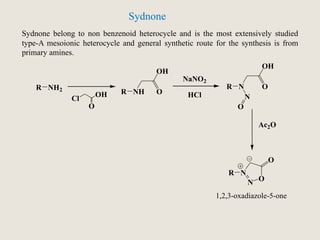
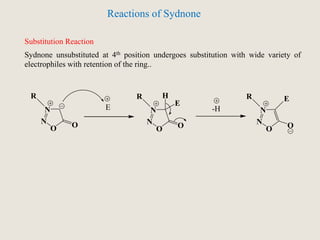
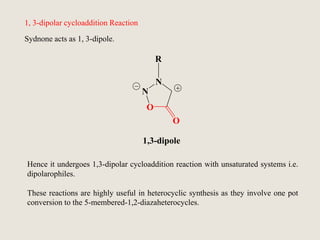
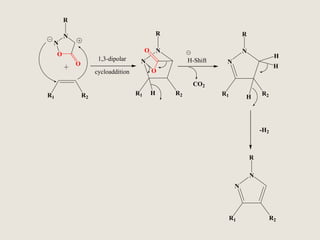
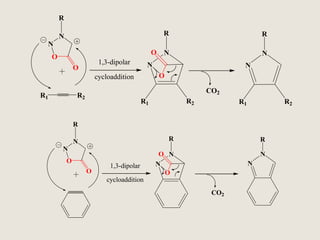
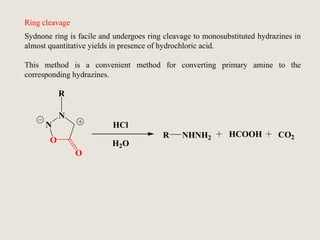
Mesoionic compounds are heterocyclic compounds with delocalized positive and negative charges that cannot be represented by any single covalent or polar structure. Sydnone and munchnone are two representative mesoionic compounds. Sydnone, the most extensively studied mesoionic compound, undergoes substitution reactions and 1,3-dipolar cycloaddition reactions. It can also undergo ring cleavage to form monosubstituted hydrazines.