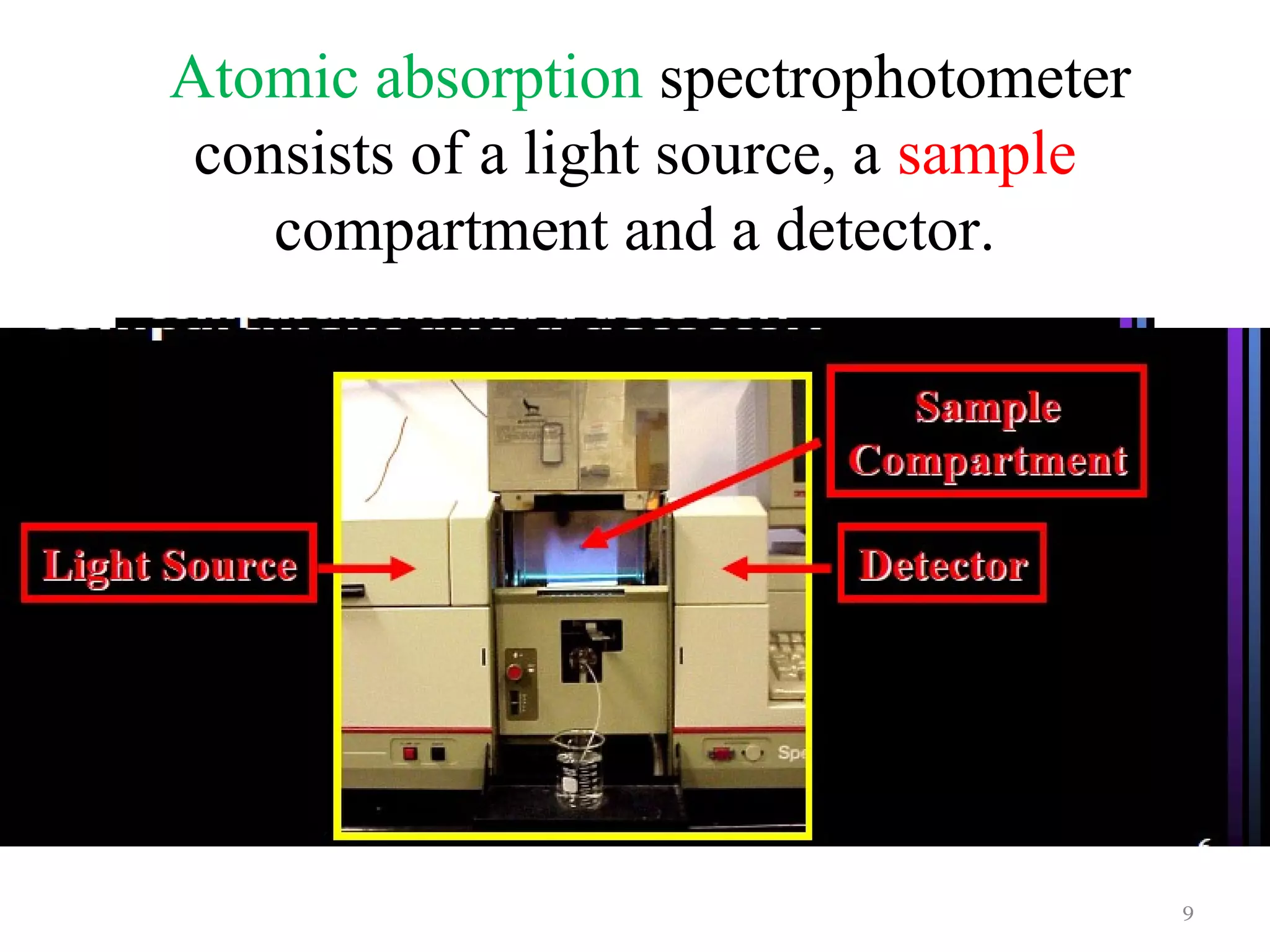
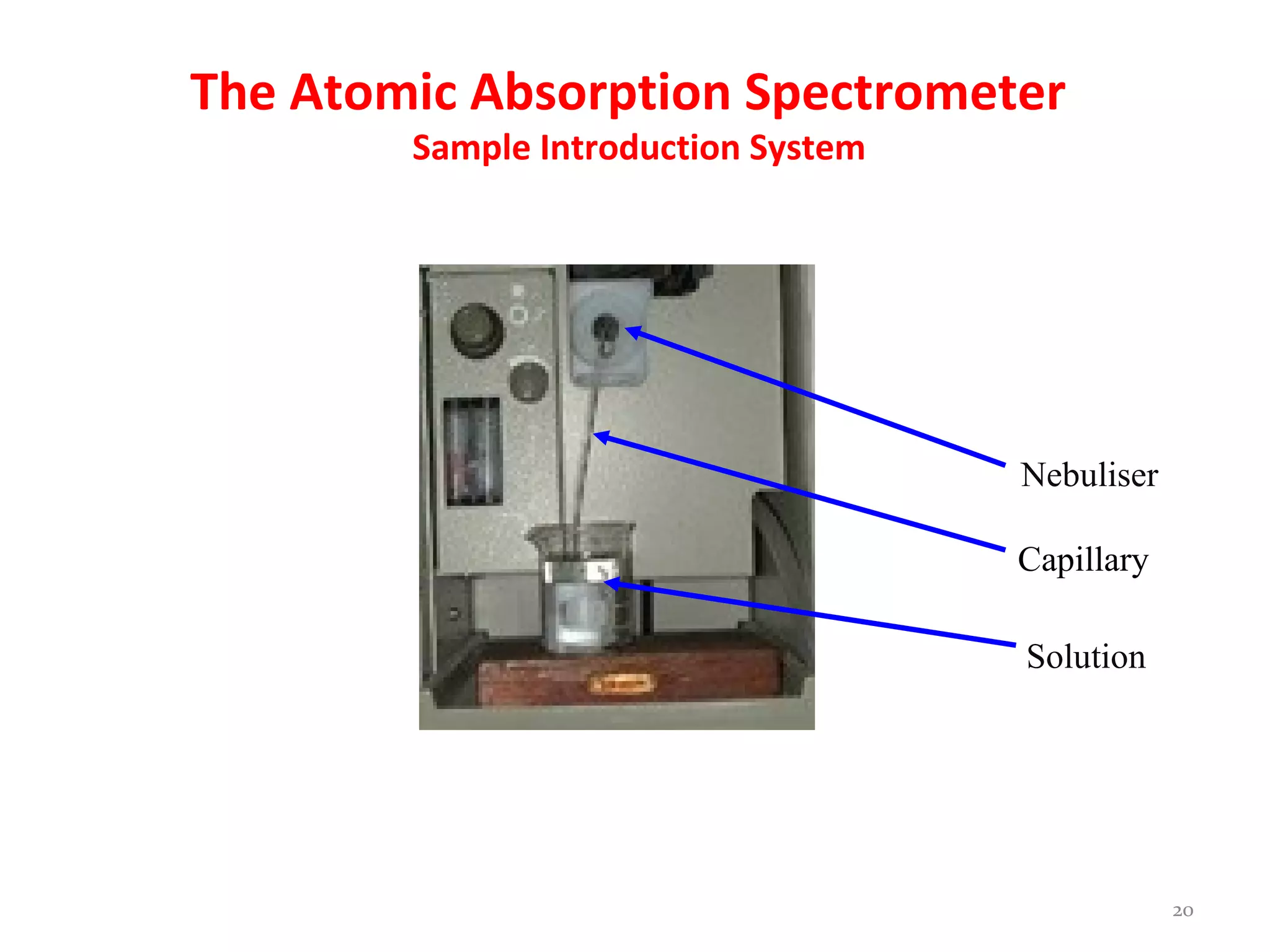
Atomic absorption spectroscopy is an analytical technique used to determine the concentration of metal elements in solutions. It works by vaporizing the sample into atoms and measuring how much light of a specific wavelength is absorbed. The technique was largely developed in the 1950s and can analyze over 70 metal elements. It uses a light source, atomizer like a flame or graphite furnace to vaporize samples, monochromator, and detector. Flame atomic absorption is common but graphite furnace offers greater sensitivity for smaller sample sizes.