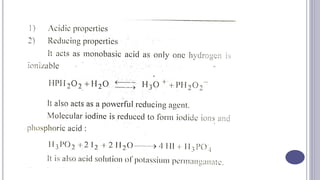
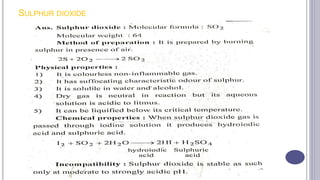
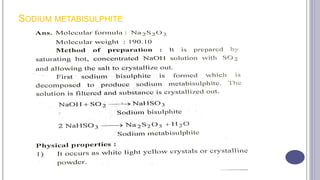
The document discusses antioxidants, which are agents used to prevent oxidation and rancidity in oils, fats, and pharmaceutical preparations. It includes classifications of antioxidants, ideal properties, and commonly used examples such as hypo phosphorous acid and sodium bisulphate. Additionally, it poses questions related to the definition and characteristics of antioxidants.