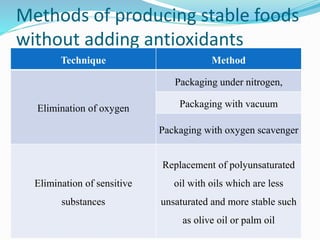
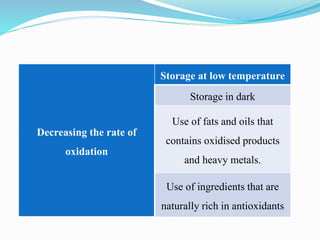
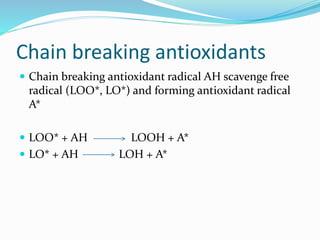
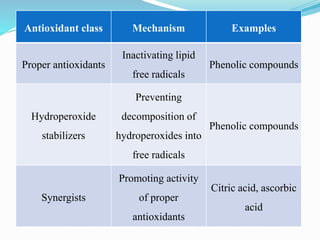
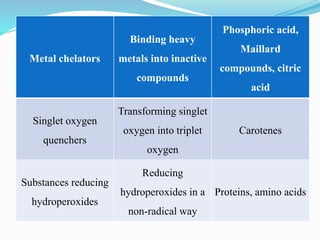
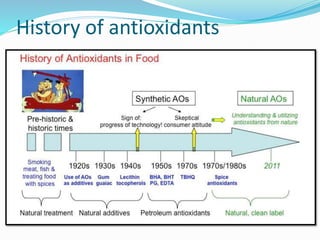
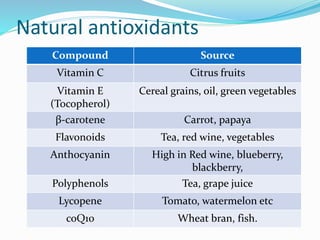
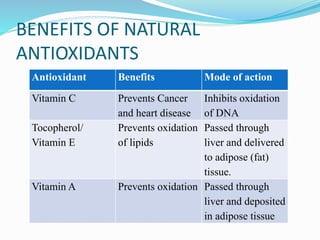
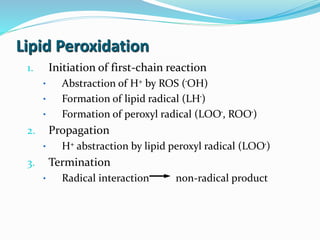
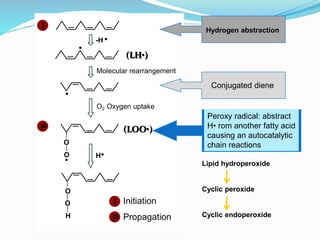
This document presents information about oxidation, free radicals, and antioxidants. It discusses how oxidation contributes to diseases and food deterioration. Free radicals are unstable molecules that can cause oxidation. Antioxidants prevent oxidation by donating electrons to free radicals. The document outlines various natural and synthetic antioxidants, how they work, and their health benefits. It also discusses regulations around approved food antioxidants and trends in antioxidant research.


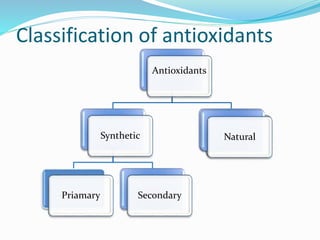
![Antioxidants
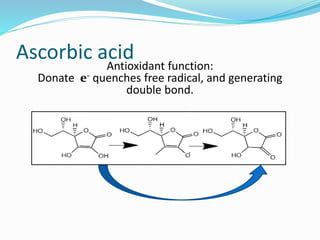
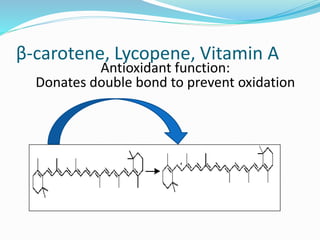
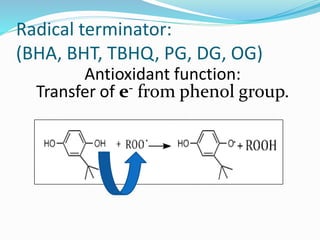
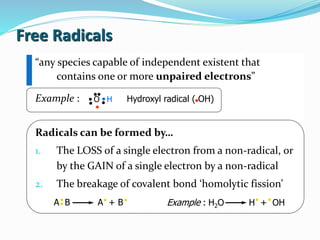
Antioxidants are the compounds that prevent oxidation.
They delay autoxidation by inhibiting formation of free
radicals
They prevent oxidation by donating electrons from their
hydroxyl [-OH] group
Free radical binds with hydroxyl group and result in stable
double bond](https://image.slidesharecdn.com/antioxidanttechnicalppt-160722101713/85/Antioxidants-in-Food-7-320.jpg)